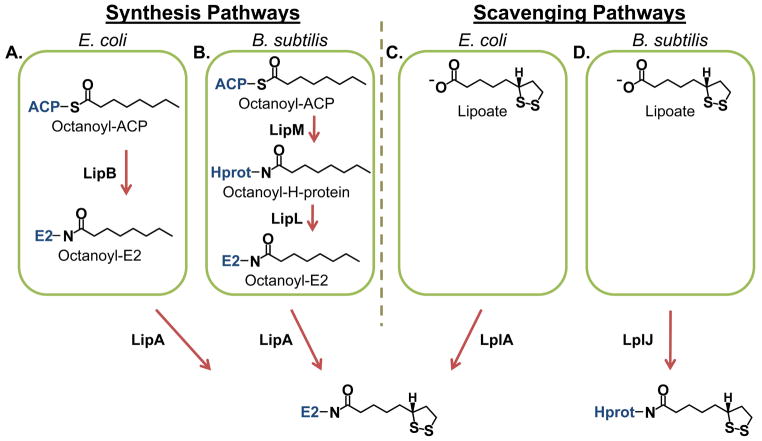
Fig. 1.
Lipoate synthesis and scavenging pathways.
(A) Lipoate synthesis as observed in E. coli. Octanoyl groups from octanoyl-ACP (acyl carrier protein) are transferred to E2 proteins (and the H-protein) by an octanoyltransferase (LipB) followed by the insertion of sulfur atoms by a lipoate synthase (LipA).
(B) Lipoate synthesis as observed in B. subtilis. Octanoyl groups are transferred specifically to the H-protein by octanoyltransferase (LipM) and are subsequently distributed to the E2 proteins by an amidotransferase (LipL). As in E. coli, sulfur insertion is catalyzed by a lipoate synthase (LipA).
(C) Lipoate scavenging as observed in E. coli. Lipoate scavenged from the external environment is attached to E2 proteins (and the H-protein) by an ATP-dependent lipoate ligase (LplA).
(D) Lipoate scavenging as observed in B. subtilis. The lipoate ligase (LplJ) has specificity for the H-protein.