Abstract
In a dyadic informed forager task, chimpanzees (Pan troglodytes) are known to exploit the knowledge of informed subordinates; however, the behavioral mechanisms they employ are unknown. It is tempting to interpret outcome measures, such as which individual obtained the food, in a cognitively richer way than the outcomes may justify. We employed a different approach from prior research, asking how chimpanzees compete by maneuvering around each other, whether they use gaze cues to acquire information from others, and what information they use in moment-to-moment decision-making. We used cross correlations, which plot the correlation between two variables as a function of time, systematically to examine chimpanzee interactions in a series of dyadic informed forager contests. We used cross correlations as a “proof of concept” so as to determine whether the target actions were contingent on, or occurred in a time-locked pattern relative to, the referent actions. A subordinate individual was given privileged knowledge of food location. As expected, an ignorant dominant followed the informed subordinate’s movement in the enclosure. The dominant also followed the subordinate’s gaze direction: after she looked at the subordinate, she was more likely to gaze towards this same direction within one second. In contrast, the subordinate only occasionally followed the dominant’s movement and gaze. The dominant also changed her own direction of movement to converge on the location to which the subordinate directed her gaze and movement. Cross correlation proves an effective technique for charting contingencies in social interactions, an important step in understanding the use of cognition in natural situations.
Keywords: social manipulation, tactics of competition, gaze following, cross correlation
Introduction
In the informed forager paradigm, a subordinate individual is shown the location of a hidden food item, and is then released into an arena to search with an ignorant dominant competitor [Menzel, 1974]. The dominant can easily exploit the foraging success of the subordinate through physical or social pressure [Baker et al., 1981; Rohwer & Ewald, 1981; Barta & Giraldeau, 1998]; thus the subordinate has a high incentive to behave in a way that prevents the dominant from finding the food. Menzel’s work, and the various subsequent replications and extensions, have raised many questions about what chimpanzees understand about what others know, how they gain information from social partners in a foraging situation, and how they flexibly manipulate opponents through tactical deception [e.g. examples in Byrne & Whiten, 1990].
The ability to follow gaze—noticing another individual’s direction of attention to a point in space and then adjusting one’s own line of regard to the same point in space [Emery 2000]—is a powerful source for gaining information from group-mates. There is a connection between the overt behavioral act of seeing and the mental state of knowing: one who witnesses an event has knowledge about it [Wimmer et al., 1988; Povinelli et al., 1999; Hare et al., 2001]. Researchers have studied the link between gaze following and knowledge attribution in chimpanzees—questioning whether chimpanzees have mental states, attribute mental states to others, and what the content of those mental states would look like. Yet clear experimental evidence of chimpanzee mental state understanding remains elusive.
Several studies have shown that non-human primates visually orient to the direction of the head, rather than body, when the two are incongruent [Kaminski et al., 2004; Shepherd & Platt, 2008] and are more successful in following head orientation than eye gaze [Povinelli & Eddy, 1996; Reaux et al., 1999; Kaplan & Rogers, 2002; Kaminski et al., 2004; Tomasello et al., 2007; Bulloch et al., 2008; Hattori et al., 2010]. Furthermore, although human observers can sometimes detect chimpanzee eye direction [Bethell et al., 2007], chimpanzee eyes have dark pigmented sclera, making eye-gaze harder to determine from the direction of the pupil/iris in the skull than in humans [Kobayashi & Kohshima, 2001]. Since chimpanzees generally follow gaze by using head- rather than eye-orientation, we used head direction as a proxy for gaze in this study.
Great apes can follow a human demonstrator’s head orientation around opaque barriers [Call et al., 1998; Tomasello et al., 1999], and can sometimes use humans’ head orientation as indicating the location of hidden food in object choice tasks. For many years it was thought that apes systematically failed at the object choice task [Povinelli et al., 1990; Call et al., 1998]. A re-evaluation of the evidence in two recent reviews suggests, however, that a cue that is presented centrally can distract subjects and indeed the proportion of positive findings in experiments with peripheral cues is higher [Lyn, 2010; Mulcahy & Hedge, 2012].
Additionally, naturalistic studies have repeatedly found that animal subjects appeared to use conspecific gaze cues to gain information [Menzel, 1974; Coussi-Korbel, 1994; Held et al., 2000, 2002, 2010; Hare et al., 2001, 2003; Hirata & Matsuzawa, 2001; Ducoing & Thierry, 2003, 2004; Bugnyar & Kotrschal, 2004; Schloegl et al., 2008b]. Furthermore, chimpanzees do have some understanding of what conspecific competitors have seen during a recent baiting procedure in an indoor holding space [Hare et al., 2000, 2001]. During these studies, a subordinate subject was shown the location of a hidden food item and competed with a dominant, who was either able or unable to see the food, and had either seen it placed in its current location or was absent during placement. Results suggested that the subordinate subjects knew whether or not the dominant knew about the food, based not only on what they could currently see, but also on what they had or had not seen during earlier baiting. Hare et al. [2000, 2001] concluded that chimpanzees “know what conspecifics do and do not see” and “know what conspecifics know.”
It has been tempting to interpret outcome measures of informed forager tests in a cognitively rich way, suggesting that chimpanzees are capable of manipulating one another in a cognitive arms race. No study, however, has specifically addressed how observed behavior, including gaze, is utilized to gain information in socially competitive situations. Though many interesting interactions between informed subordinates and ignorant dominants have been reported, the interactions that observers described as happening were not supported by statistical analyses and have generally been regarded as anecdotes in the pejorative sense [Bernstein, 1988]. For example, reporting the mean percentage of food items that either competitor obtained does not describe the interaction that led to that outcome; stating whether the competitors could see each other at the time of baiting does not reveal how either individual used visual information to modify her own competitive tactic; and in particular, there is a lack of an appropriate statistic to handle data on gaze interactions.
Our aim was to adapt cross correlations, a method developed within neurology, to analyze familiar data in a novel way, and we present our results as “proof of concept” for using this method to settle long-standing questions in primatology and the behavioral field at large. In neurophysiology, cross-correlograms, which plot the correlation between two variables as a function of time [Oram et al., 2001], are commonly used to report the precise temporal relationship of activity between two neurons with one spike train chosen as the referent and the other as the target [Perkel et al., 1967; Aertsen et al., 1989]. In this study, we used cross-correlograms to examine the behavioral interactions of two chimpanzees, so as to determine whether the target actions were contingent on, or occurred in a time-locked pattern relative to, the referent actions. We present a more detailed analysis of gaze following during competitive dyadic interactions; we therefore used cross correlations in order to determine which gaze cues elicit movement following, whether both competitors follow the other’s gaze, and how gaze following interacts with movement patterns.
Method
Study site and subjects
We collected data from October 2010 to August 2011 from one pair of captive, unrelated, adult female chimpanzees (P. troglodytes), at the Yerkes National Primate Research Center field station in Lawrenceville, Georgia, USA. Missy (aged 18) was subordinate to Rita (23), as determined by three indoor dyadic food competitions. Research complied with protocols approved by the Institutional Animal Care and Use Committee (IACUC) and adhered to the legal requirements of the United States. The research adhered to the American Society of Primatologists (ASP) principles for the ethical treatment of non-human primates.
The subjects lived in the same social group of 11 individuals, housed in an outdoor enclosure (24 × 30 m) with two indoor areas. The outdoor enclosure contained a three-level climbing structure with ladders and ropes, as well as several tires, kegs, barrels, and other enrichment objects. The indoor areas comprised six holding spaces (3 × 3 × 3 m each) in the ‘Bedroom’ and five holding spaces (1.74 × 1.74 × 1.74 m each) in the ‘Testing Room.’ All chimpanzees were fed twice a day with chow, fruit and vegetables, and water was available ad libitum. Chimpanzees were not deprived of food at any time and were not subject to any invasive procedure.
Experimental procedure
Non-participating chimpanzees were locked into the ‘Bedroom’ area and could not witness the experiment. During baiting, the informed subordinate was held in a holding space in the ‘Testing Room’ with visual access into the outdoor enclosure. The ignorant dominant was held in an adjacent holding space with no visual access to the outdoor enclosure. Each chimpanzee, however, could see her competitor through a mesh panel (72 × 52 cm).
During baiting, the experimenter (KH) entered the outdoor enclosure, which was void of chimpanzees, and attracted the subordinate’s attention through the window with a banana. While the subordinate was watching, KH hid the banana in one of sixteen pre-determined hiding locations. We treated the outdoor enclosure as composed of four approximately equal quadrants, each with four hiding places. The baiting schedule cycled through the four quadrants in a counterbalanced order. After baiting, KH left the compound and released first the dominant and then the subordinate into the enclosure, with a delay of approximately three seconds between the hydraulic doors opening fully. The pair was tested in 24 trials.
Data coding
Each trial was video recorded from opposite sides of the enclosure. An unmanned video camera (Panasonic PV-GS320) on the Viewing Tower was set to record the majority of the enclosure’s area. KH recorded from the Office Tower (Sony DCR-HC52 and/or Canon Vixia HF100), zooming in to frame the two competing individuals and record their head orientations and gaze interactions.
Videos were coded using Noldus Observer XT 9 software (Noldus Information Technology, Inc., Leesburg, Virginia). Continuous variables were recorded using instantaneous focal sampling every second. The subject’s location was coded as which of the four quadrants she was currently in. Similarly, direction of movement, head and body orientation were coded in terms of quadrant (zero if not moving). Direction of movement and gaze relative to the opponent (towards or away) and relative to the bait (towards or away) were recorded. In addition, point variables were recorded using all-occurrence sampling [Altmann, 1974] for behavior categories without duration, e.g. ‘change direction’ and ‘seize food.’
We defined gaze following as: “individual A is gazing towards Quadrant X, then individual B looks at A, after which B also looks towards Quadrant X.” We defined following off-axis gaze as: “individual A changes her gaze direction from Quadrant X to Quadrant Y while in view of individual B, after which B also looks towards Quadrant Y.” The individual A that changes her gaze should be in B’s potential field of vision (i.e., not behind A, or hidden behind a barrier), but B does not necessarily have to be looking directly at A at the time A changes her gaze. We defined movement convergence as, “individuals A and B are in different Quadrants X and Y and walking towards the same quadrant.”
Statistical analysis
To construct a correlogram, the behaviors of interest are converted into a binary time series, with 1 indicating the presence of the behavior, 0 the absence of the behavior. Each trial is divided into a series of non-overlapping time bins, with the bin duration short enough so that only one occurrence of a particular behavior occurs within a single bin. Examination of the video recordings suggested that bins with a width of one second met this requirement. Thus a time series was coded using a binary variable, a value occurring every second. For behaviors initially coded as, for example ‘which quadrant the animal was in,’ four proxy codes were created, one for each quadrant (‘in quadrant one (1/0)’; ‘in quadrant two (1/0)’, etc.). These binary series could then be combined to form a new series of binary values: ‘both the dominant (D) and subordinate (S) in same quadrant’ is constructed using the logical statement
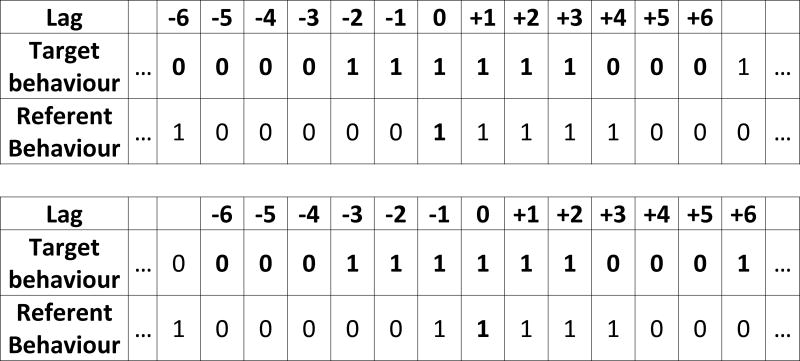
The series have a natural alignment at the start of the trial, when the doors to the external enclosure were opened. One binary series is designated the referent behavior, a second as the target behavior (Fig.1). A bin in the referent time series is then chosen to represent t=0. The values of the target behavior at different lags relative to the referent series can then be read (-6 to +6 seconds in Fig. 1, upper). The next time bin in the referent series is then selected as the t=0 bin and the process repeated (Fig. 1, lower). Pearson’s correlation of the pairs of values at each lag (in this publication, -20 to +20 seconds) is then calculated. That is, the correlation coefficient is an indicator of the effect size of the contingency between the two variables (the correlation coefficient of two binary variables is the same as the phi-coefficient obtained from the associated contingency table).
Figure 1. Schematic showing the calculation of the cross-correlogram.
One of two binary time series is labeled as the referent, the other the target. A single time bin in the referent series is taken and the value of the target time series at different lags (here -6 to +6 time bins) noted. Each pair of values at a given lag is taken as an (X,Y) pairing for the calculation of the correlation coefficient. In the example above, at lag -3 the pairs are (0,0) and (1,0); at lag 0 the pairs are (1,1) and (1,1) while at lag +3 the pairs are (1,1) and (0,1).
If the behavior of one animal follows that of another, then the value of the correlation between the two behaviors will increase at the relevant lag. Note there is a symmetry with respect to which series is taken as the referent and the sign (+ or -) of the lag: the correlation values are the same, but at lags with reversed sign. The trials finished at varying times and thus there are data points where the time between referent behavior and target behavior is more or less than 20 seconds, the range depicted in the figures in this publication. Therefore, the amount of data in the calculations decreases as the lag increases. As the correlation coefficient is an unbiased estimate, its value is not impacted, except in the sense of increased uncertainty of the estimate. This is taken into account with the evaluation of statistical significance. The reference point is not ‘at the start’ of behavior X, but rather ‘while doing behavior X.’ For example, if one subject starts to follow another, at some point in the future, they stop moving or the subject decides not to follow. In those scenarios, the correlation coefficient decreases over time.
While it is tempting to evaluate the significance of the correlation using standard statistical techniques, the results are unreliable and result in a large excess of type 1 errors [Oram et al., 1999]. There are multiple sources of correlation in behavioral time series. Some of these are obvious, such as the autocorrelation of behavior: once started many behaviors such as walking tend to continue for several seconds. A second source of correlation within trials comes from violation of the distribution of counts from Poisson [Oram et al., 1999, 2001]. Any source of auto-correlation will, in-turn, impact on the cross correlation between behaviors [Oram et al., 2001].
To accurately evaluate the significance of the correlation coefficients we compare the observed correlation coefficient with the distribution of its expected value after disrupting potential sources of correlation. In the between-trial shuffle the trial numbers are randomized. For example, the data from trial 1 of one animal could be paired with the data from trial 6 (rather than the trial 1 data) of the second animal. This shuffle disrupts the cross-trial relationship and can thus be thought of as disrupting coarse temporal relationships of behavior while maintaining any fine temporal relationship. Alternatively, in a within-trial shuffle, the values of time bins of each series within a trial are randomly swapped. This maintains the number of 1’s and 0’s in each trial and any coarse temporal correlation between different trials, selectively disrupting fine temporal correlation. Finally, a combined within- and between-trial shuffle could be performed. This dual shuffle is equivalent to using conventional significance evaluation and results in disruption of all sources of correlation.
The choice of the most appropriate shuffle is not trivial. The impact of autocorrelation on the values in the cross-correlogram can be less than the impact of slow variations between trials [e.g. Brody, 1999; Oram et al., 1999, 2001]. A further complication arises if, as here, the behavior of interest is itself contingent on another behavior. For example, suppose we want to know the extent to which one animal tends to walk towards the location a second is looking at. In such a scenario the second animal determines the ‘location.’ If the tendency to walk towards where another was looking were high, you would see the behavior in most trials. Between-trial shuffles might not, therefore, disrupt the correlation of interest. Given the potentially large impact of coarse temporal correlation on the fine temporal structure, combined with the fact that here we are specifically interested in fine temporal relationships in behavior (i.e. does the behavior of one chimpanzee influence the behavior of second chimpanzee at a second-by-second time scale), we use the within-trial shuffle to provide a measure of significance (labeled ‘Noise’ on the figures). That is, we obtain a test of significance of the observed correlogram given the correlations due to the fine temporal relationships (except in one case, Fig.3 for which the between-trial shuffle is more appropriate given that the behavior is directed at absolute locations, i.e. Quadrants 1-4, rather than relative to the competitor chimpanzee). For the within-trial shuffle, the number of unique shuffles is very large and hence 10,000 random shuffles were used to develop an estimate of the correlogram under the null hypothesis that there was no fine temporal relationship between the behaviors of the chimpanzees. We note that the patterns of significance levels when the observed values are compared to the between-trial shuffle follow a similar pattern [Brody, 1999; Oram et al., 1999, 2001].
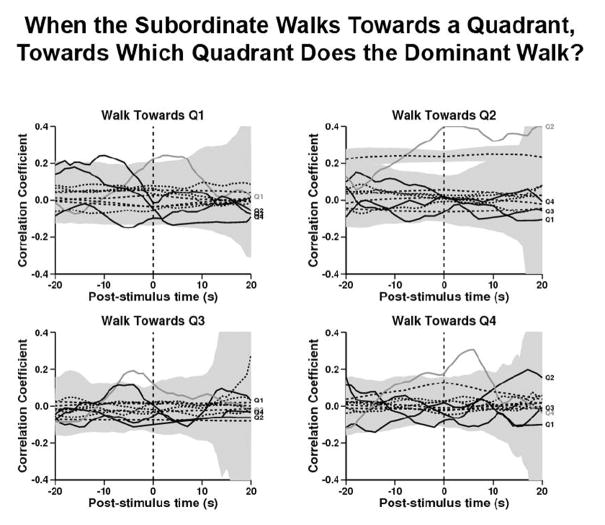
Figure 3. When the Subordinate walks towards a quadrant, towards which quadrant does the Dominant walk?
In each panel, the referent behavior at time= 0 is the subordinate walking towards the quadrant of that panel (the layout corresponds to the physical layout of the enclosure). The lighter-colored solid line in each panel represents the cross correlation of the dominant walking towards the same quadrant as the subordinate. The three solid black lines in each panel represent the cross correlation of the dominant walking towards each of the other three quadrants. The dotted lines with the gray spread represent the within- and between-trial shuffled controls and their standard errors of the mean. For example, in the top left panel, the referent behavior at time= 0 is the subordinate walking towards Q1 and it is correlated with the target behavior of the dominant walking towards Q1, and this relationship is plotted with a lighter gray line. The black lines show the cross correlations of the subordinate walking towards Q1 with the dominant walking towards Q2, Q3, and Q4. After the subordinate started walking towards Q1 (p<0.05), Q2 (p<0.05), and Q4 (p<0.05), the dominant walked towards the same quadrant as the subordinate significantly more than expected from the between-trial shuffled control. The dominant walked towards Q3 before the subordinate (ns).
The expected distribution of the correlation coefficient is not Gaussian when the expected value deviates from zero. Fisher’s transform converts correlation coefficients such that they have a normal distribution independent of the value of the coefficient. Thus, all values from the shuffles were Fisher transformed. This enabled the calculation of the t-value of the Fisher transformed observed correlation to be evaluated.
How long this sort of analysis takes will depend on the number of variables being correlated, and that in turn depends on whether a specific question(s) is being answered or a discovery method is needed. With a precise question to be answered, computationally less intensive methods may be as effective. In the more general case, where it is not straightforward to pre-specify the ‘key’ variables to study (including temporal relationships), cross-correlation allows analysis of many variables over variable timescales. We suggest that cross-correlations will be best used to investigate whether behavioral contingencies exist between subjects, in part because of examining multiple timescales and relationships between multiple variables.
Results
This study was based on the expectation that an ignorant dominant chimpanzee (D) would be able to use the behavior of an informed subordinate (S) in order to find hidden food, as in previous studies. We therefore examined this expectation first, before going on to investigate how. Overall, S obtained 18 of 24 bananas, and D obtained the other 6 (D obtained her first banana on trial 11). The results should therefore be familiar, but are presented in a new way.
Does the dominant use the subordinate’s movement cues?
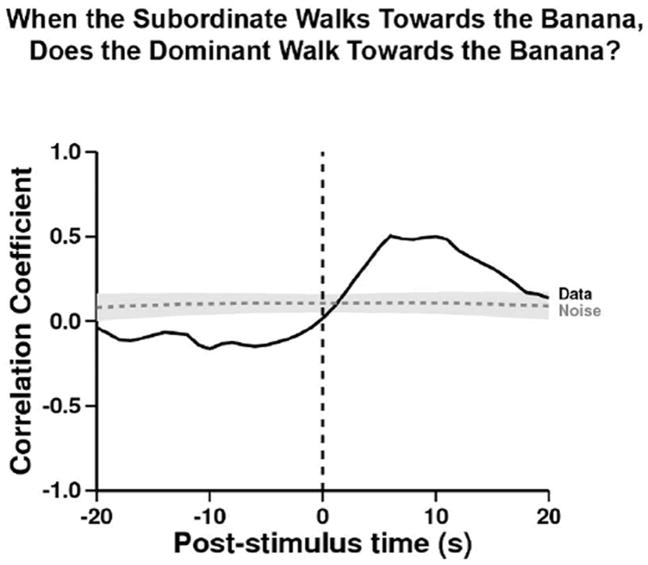
We tested the hypothesis that the ignorant D was able to use S’s movement to guide her own by asking “When S walks towards the bait, does D walk towards the bait?” The correlogram indicates a clear contingency (Figure 2). Recall that at time= 0, the action of “S walks towards the bait” is indicated, and in the 20 seconds before and after, we plot the correlation of that with “D walks towards the bait.” Thus, any positive correlation after t= 0 represents e.g., both subjects walking towards the bait (whereas a negative correlation would indicate e.g. that S walked but D did not).
Figure 2. When the Subordinate walks towards the banana, does the Dominant walk towards the banana?
Referent behavior: subordinate walking towards the bait. Target behavior: dominant walking towards the bait. Before S was walking towards the bait, D was walking towards the bait significantly less than expected from the within-trial shuffled control (peak t= -6, r= -0.15; –log-likelihood = 10.7, p<0.05). After S started to walk towards the bait, D walked towards the bait significantly more than expected from the within-trial shuffled control (peak at time= +6, r= 0.50, n= 194; –log-likelihood = 21.4, p<0.05). The gray area labelled ‘Noise’ is the estimated standard error of the calculated correlation coefficient (see main text for details).
Before S began walking towards the bait, D was unlikely to be walking towards the bait (correlation coefficients less than zero, p<0.05 for lags -19 to -1 second). However a few seconds after S began walking towards the bait, D also tended to walk towards the bait (correlation greater than zero, p<0.05 for lags +3 to +17 seconds). Note that the data used do not include the time when the bait is found: thus, D is not simply responding to seeing S retrieve the bait (See Statistical analysis section above for details on how variable trial length affects the correlation coefficient).
We also examined whether the particular location at which the bait was present affected these patterns, but found that D was more likely to walk towards the same general quadrant of the enclosure as S, after S first walked towards it, regardless of the location of the hidden bait (Fig.3).
Does the dominant use the subordinate’s gaze cues?
D could simply be following S’s movement towards the bait, but there are subtler cues to the intentions of another that might not only enable D to follow S, but allow for more efficient (shorter path) following, by anticipation of S’s movements. In particular, we ask if gaze following was used to guide D’s search. We found that after D looked at S, both chimpanzees gazed towards the same quadrant significantly more than expected over a period of several seconds (Fig.4, upper). In contrast, after S looked at D, S tended to look in a direction away from where D was looking (Fig.4, lower).
Figure 4. When the Dominant looks at the Subordinate, do they both gaze towards the same quadrant?
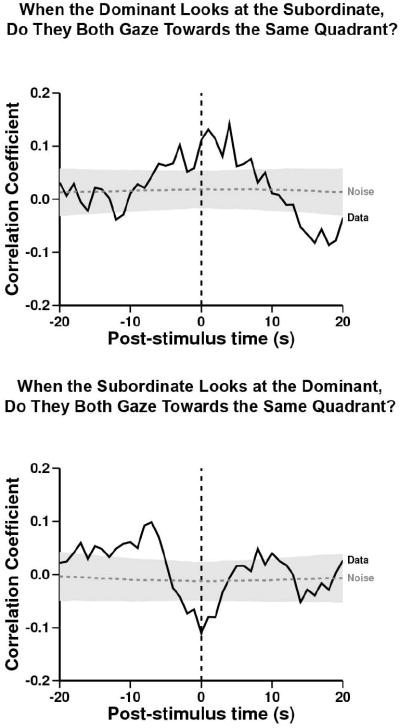
Referent behavior: dominant looking at subordinate. Target behavior: both chimpanzees gazing towards the same quadrant. After the dominant looked at the subordinate, both chimpanzees gazed towards the same quadrant significantly more than expected from the within-trial shuffled control (peak at time= +4, r= 0.14, n= 831; –log-likelihood= 7. 1, p<0.05). When the Subordinate looks at the Dominant, do they both gaze towards the same quadrant? Referent behavior: subordinate looking at dominant. Target behavior: both chimpanzees gazing towards the same quadrant. At the moment that the subordinate looked at the dominant, both chimpanzees gazed towards the same quadrant significantly less than expected from the within-trial shuffled control (peak at time= 0, r= -0.11, n= 855; –log-likelihood= 5.0, p<0.05). Before the subordinate looked at the dominant, both chimpanzees gazed towards the same quadrant significantly more than expected from the within-trial shuffled control (peak at time= -7, r= 0.10, n= 708; –log-likelihood= 5.2, p<0.05).
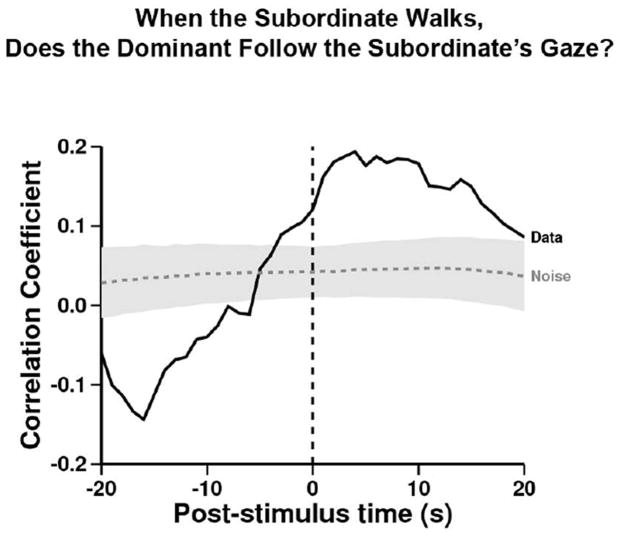
This analysis, however, is problematic since gaze following may be confounded by movement direction. To address this concern, we tested whether D followed S’s gaze more while S was walking than static (Fig.5). D was unlikely to follow S’s gaze while S was not walking (before t= 0); but after S began to walk, D followed S’s gaze significantly more than expected. This highlights that D was motivated to follow S’s gaze specifically while S appeared to be searching for the hidden food.
Figure 5. When the Subordinate walks, does the Dominant follow the Subordinate’s gaze?
Referent behavior: subordinate walking. Target behavior: dominant looks at subordinate and then both chimpanzees gaze towards the same quadrant. D followed S’s gaze only after S began walking significantly more than expected from the within-trial shuffled control (peak at time= +4, r= 0.19, n= 770; –log-likelihood= 11.2, p<0.05).
To further disambiguate gaze direction and movement direction, we examined when one chimpanzee’s direction of gaze (as indicated by her head direction) was off-axis from the direction of her body. We therefore asked, “When S gazes off-axis, does D gaze to the same quadrant towards which S changed her gaze direction?” D did indeed follow S’s gaze in this case (Fig.6, upper): before S gazed off-axis (t<0), D was not looking where S was looking, and when S changed her gaze so that it was off-axis from her body, D looked where S was looking. This indicates D’s sensitivity to the incongruence in S’s head and body directions. We found a similar result in the reverse scenario: S also followed D’s gaze when D gazed in a different direction from the direction of her body (Fig.6, lower). Both subjects were sensitive to the other’s gaze direction when it was different from the direction of the other’s body.
Figure 6. When the Subordinate gazes off-axis, does the Dominant follow the Subordinate’s gaze?
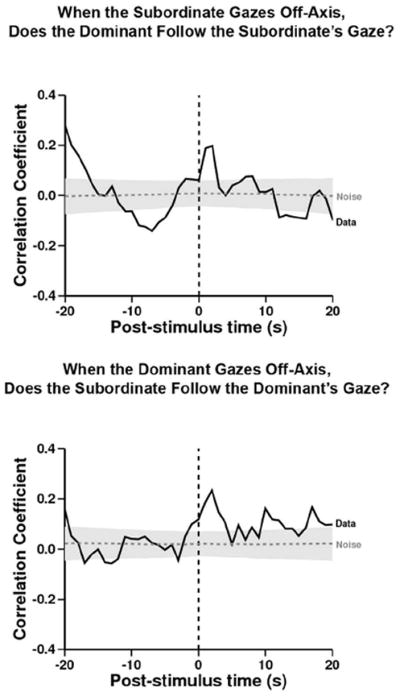
Referent behavior: S changes her gaze to off-axis while in view of D. Target behavior: D follows S’s gaze. In this case, D was likely to follow S’s change in gaze direction significantly more than expected from the within-trial shuffled control (peak at time= +2, r= 0.20, n= 447; –log-likelihood= 7.8, p<0.05). When the Dominant gazes off-axis, does the Subordinate follow the Dominant’s gaze? Referent behavior: D changes her gaze to off-axis while in view of S. Target behavior: S follows D’s gaze. In this case, D was likely to follow S’s change in gaze direction significantly more than expected from the within-trial shuffled control (peak at time= +2, r= 0.23, n= 355; –log-likelihood= 10.4, p<0.05).
Does the dominant modify her search according to the subordinate’s behavior?
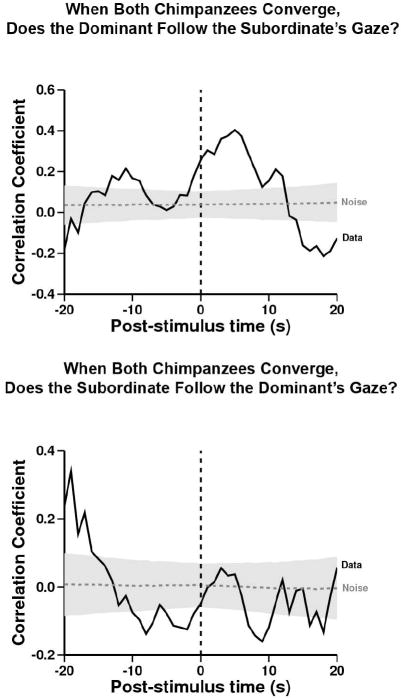
D was evidently capable of following both S’s movement and, more subtly, S’s gaze (when it is on- and off-axis from the direction of her body). We next asked if D modified her own searching behavior after these interactions. To test the hypothesis that D used S’s gaze direction as a cue to determine the direction that S would walk, and that D would modify her own movement to end up at the same location as S, we analyzed how each subject behaved when both chimpanzees converged on the same area (See definition in Methods). Here we found evidence that D looked at S, and then towards the same quadrant where S gazed (presumably S was looking ahead to her destination), and D walked towards the same quadrant that S was gazing and moving (Fig.7, upper). In all convergences, D waited until S started to walk before D started to walk. In contrast, S did not tend to look at D and then towards the same quadrant where D gazed, while converging (Fig.7, lower).
Figure 7. When both chimpanzees converge, does the Dominant follow the Subordinate’s gaze?
Referent behavior: both chimpanzees converging. Target behavior: dominant following the subordinate’s gaze. When both chimpanzees were in different quadrants and moving towards the same quadrant, the dominant looked at the subordinate and then both chimpanzees gazed towards the same quadrant significantly more than expected from the within-trial shuffled control (Peak at time= +5, r= 0.40, n= 223; –log-likelihood= 13.9, p<0.05). When both chimpanzees converge, does the Subordinate follow the Dominant’s gaze? Referent behavior: both chimpanzees converging. Target behavior: subordinate following the dominant’s gaze. After both chimpanzees were in different quadrants and moving towards the same quadrant, the subordinate looked at the dominant and then both chimpanzees gazed towards the same quadrant significantly less than expected from the within-trial shuffled control (Peak at time= +9, r= -0.16, n= 188; –log-likelihood= 3.4, p<0.05).
Discussion
Our results provide detailed evidence that chimpanzees use visual information to modify their competitive tactics. In an attempt to show quantitatively the “continuous feedback” between subjects that previous published studies have merely described as happening [Menzel, 1974; Coussi-Korbel, 1994; Held et al., 2002; Hirata & Matsuzawa, 2001; Ducoing & Thierry, 2003, 2004; Bugnyar & Kotrschal, 2004], we systematically investigated with cross correlations the specific behavioral contingencies during chimpanzee competition. We found that the dominant D consistently followed the subordinate S’s movement, both towards the bait and towards absolute locations in their enclosure (Quadrants 1-4). These findings suggest that D attempted to exploit S by physically following S around their enclosure: when S walked towards the hidden bait, D also walked towards it and D changed her following tactics as the interaction developed.
We observed D using S’s gaze, as indicated by head orientation, including when gaze direction was off-axis from the body orientation. If gaze direction can reveal information about what the chimpanzee knows, we would expect D to have followed S’s gaze in order to seek information from her because only S had privileged knowledge of the location of a hidden food item. D was likely to gaze towards the same quadrant as S, after first looking at S, which we consider evidence of gaze following. In contrast, S was less likely to follow D’s gaze, an internal control indicating differential behavior that at least corresponds with the presumed knowledge state of the animals. Both chimpanzees were sensitive to changes in the direction of their competitor’s gaze when the competitor’s head direction was off-axis from her body direction. Tomasello et al. [1998] and Hare et al. [2000] reported that chimpanzees are able to follow the gaze of a conspecific, and our data lend additional substance to this claim.
Moreover, our results show how gaze following is combined with attention to a competitor’s behavior. In general, D was more likely to follow S’s gaze when S was walking, indicating attention to her behavior. Specifically, we analyzed each subject’s use of gaze following while converging, when the two chimpanzees walked towards the same location from two different starting locations. D followed S’s gaze while both converged, whereas S was not likely to follow D’s gaze. These data are consistent with laboratory findings that chimpanzees can follow gaze geometrically and around barriers [Tomasello et al., 1999; Okamoto-Barth et al., 2007]: D used S’s gaze to adjust her own movement, and thereby walked towards S’s predicted destination, perhaps improving her foraging success. That S did not similarly follow D’s gaze indicates that S was either focused on reaching her goal of finding the hidden bait, and/or was less motivated to try to gain information from D, who was ignorant of the bait’s location. Thus, our results lend support to the interpretation that D was extrapolating S’s movement, and not the other way around.
We have shown that chimpanzee subjects are able to use conspecific gaze cues in an ‘ecologically valid’ competitive context [Matheson et al., 1998; Hare, 2001; Hare & Tomasello, 2004]. While our analysis of a subject’s movement and gaze monitoring cannot prove that ‘D knew that S knew where the food was hidden,’ it provides insight into the specific competitive tactics used, tactics presumably developed on the basis of prior experience and appropriately tuned to how the opponent behaves from moment to moment.
Our study illustrates the value of cross correlation as a technique for behavioral studies and the field of ethology in particular. Cross correlations can show broad relationships between subjects, such as various movement following patterns and how they change over time. In addition, cross correlations can pinpoint specific contingencies, such as the relationship between looking at the competitor and then immediately gazing towards the same direction as the competitor. They have brought to light the most detailed picture of how chimpanzees compete and employ different movement and gaze following tactics in the informed forager paradigm. The results presented here allow for a better understanding of gaze following in relation to information acquisition, and how gaze cues (and in principle any recorded behavior) can be used to modify one’s own competitive tactics.
Acknowledgments
This project was supported by the Living Links Center of the Yerkes National Primate Research Center and Emory University College funds. The YNPRC receives support from NIH’s National Center for Research Resources P51RR165 (currently supported by the Office of Research Infrastructure Programs/OD P51OD11132). KH was supported by the Janet T. Anderson Trust, the Scottish Overseas Research Student Award Scheme, and a studentship from the University of St Andrews School of Psychology. MWC was supported by the FIRST program (NIH/NIGMS (USA) IRACDA grant number K12 GM000680). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. Victoria Horner and J. Devyn Carter for assistance and advice, and Dr. Andrew Whiten for kindly allowing us to use his copy of the Noldus Observer program, license number OB050-03166. We thank the care, colony management, and veterinary staffs of YNPRC, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
References
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. Journal of Neurophysiology. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–266. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Baker MC, Belcher CS, Deutsch LC, Sherman GL, Thompson DB. Foraging success in junco flocks and the effects of social hierarchy. Animal Behaviour. 1981;29:137–142. [Google Scholar]
- Barta Z, Giraldeau L-A. The effect of dominance hierarchy on the use of alternative foraging tactics: a phenotype-limited producing-scrounging game. Behavioural Ecology and Sociobiology. 1998;42:217–223. [Google Scholar]
- Bernstein IS. Metaphor, cognitive belief, and science [Peer commentary on “Tactical deception in primates” by Whiten A, Byrne RW] Behavioral and Brain Sciences. 1988;11:233–273. [Google Scholar]
- Bethell EJ, Vick S, Bard KA. Measurement of eye-gaze in chimpanzees (Pan troglodytes) American Journal of Primatology. 2007;69:562–575. doi: 10.1002/ajp.20376. [DOI] [PubMed] [Google Scholar]
- Brody CD. Correlations without synchrony. Neural Computation. 1999;11:1537–1551. doi: 10.1162/089976699300016133. [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Kotrschal K. Leading a conspecific away from food in ravens (Corvus corax)? Animal Cognition. 2004;7:69–76. doi: 10.1007/s10071-003-0189-4. [DOI] [PubMed] [Google Scholar]
- Bulloch MJ, Boysen ST, Furlong EE. Visual attention and its relation to knowledge states in chimpanzees, Pan troglodytes. Animal Behaviour. 2008;76:1147–1155. [Google Scholar]
- Byrne RW, Whiten A. Tactical deception in primates: the 1990 database. Primate Report. 1990;27:1–101. [Google Scholar]
- Call J, Hare BA, Tomasello M. Chimpanzee gaze following in an object-choice task. Animal Cognition. 1998;1:89–99. doi: 10.1007/s100710050013. [DOI] [PubMed] [Google Scholar]
- Coussi-Korbel S. Learning to outwit a competitor in mangabeys (Cercocebus torquatus torquatus) Journal of Comparative Psychology. 1994;108:164–171. doi: 10.1037/0735-7036.108.2.164. [DOI] [PubMed] [Google Scholar]
- Ducoing AM, Thierry B. Withholding information in semifree-ranging tonkean macaques (Macaca tonkeana) Journal of Comparative Psychology. 2003;117:67–75. doi: 10.1037/0735-7036.117.1.67. [DOI] [PubMed] [Google Scholar]
- Ducoing AM, Thierry B. Following and joining the informed individual in semifree-ranging tonkean macaques (Macaca tonkeana) Journal of Comparative Psychology. 2004;118:413–420. doi: 10.1037/0735-7036.118.4.413. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience & Biobehavioural Reviews. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Hare B. Can competitive paradigms increase the validity of experiments on primate social cognition? Animal Cognition. 2001;4:269–280. doi: 10.1007/s100710100084. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Agnetta B, Tomasello M. Chimpanzees know what conspecifics do and do not see. Animal Behaviour. 2000;59:771–785. doi: 10.1006/anbe.1999.1377. [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know? Animal Behaviour. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. [DOI] [PubMed] [Google Scholar]
- Hare B, Addessi E, Call J, Tomasello M, Visalberghi E. Do capuchin monkeys, Cebus apella, know what conspecifics do and do not see? Animal Behaviour. 2003;65:131–142. [Google Scholar]
- Hare B, Tomasello M. Chimpanzees are more skilful in competitive than in cooperative cognitive tasks. Animal Behaviour. 2004;68:571–581. [Google Scholar]
- Hattori Y, Kano F, Tomonaga M. Differential sensitivity to conspecific and allospecifc cues in chimpanzees and humans: a comparative eye-tracking study. Biology Letters. 2010;6:610–613. doi: 10.1098/rsbl.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held S, Mendl M, Devereux C, Byrne RW. Social tactics of pigs in a competitive foraging task: The ‘informed forager’ paradigm. Animal Behaviour. 2000;59:569–576. doi: 10.1006/anbe.1999.1322. [DOI] [PubMed] [Google Scholar]
- Held S, Mendl M, Devereux C, Byrne RW. Foraging pigs alter their behaviour in response to exploitation. Animal Behaviour. 2002;64:157–165. [Google Scholar]
- Held SDE, Byrne RW, Jones S, et al. Domestic pigs, Sus scrofa, adjust their foraging behaviour to whom they are foraging with. Animal Behaviour. 2010;79:857–862. [Google Scholar]
- Hirata S, Matsuzawa T. Tactics to obtain a hidden food item in chimpanzee pairs (Pan troglodytes) Animal Cognition. 2001;4:285–295. doi: 10.1007/s100710100096. [DOI] [PubMed] [Google Scholar]
- Itakura S, Tanaka M. Use of experimenter-given cues during object-choice tasks by chimpanzees (Pan troglodytes), an Orangutan (Pongo pygmaeus), and human infants (Homo sapiens) Journal of Comparative Psychology. 1998;111:119–126. doi: 10.1037/0735-7036.112.2.119. [DOI] [PubMed] [Google Scholar]
- Itakura S, Agnetta B, Hare B, Tomasello M. Chimpanzee use of human and conspecific social cues to locate hidden food. Developmental Science. 1999;2:448–456. [Google Scholar]
- Kaminski J, Call J, Tomasello M. Body orientation and face orientation: two factors controlling apes’ begging behavior from humans. Animal Cognition. 2004;7:216–223. doi: 10.1007/s10071-004-0214-2. [DOI] [PubMed] [Google Scholar]
- Kaminski J, Call J, Tomasello M. Chimpanzees know what others know, but not what they believe. Cognition. 2008;109:224–234. doi: 10.1016/j.cognition.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Rogers LJ. Patterns of gazing in orangutans (Pongo pygmaeus) International Journal of Primatology. 2002;23:501–526. [Google Scholar]
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: Comparative studies on external morphology of the primate eye. Journal of Human Evolution. 2001;40:419–435. doi: 10.1006/jhev.2001.0468. [DOI] [PubMed] [Google Scholar]
- Lyn H. Environment, methodology, and the object choice task in apes: evidence for declarative comprehension and implications for the evolution of language. Journal of Evolutionary Psychology. 2010;8:333–349. [Google Scholar]
- Matheson M, Cooper M, Weeks J, Thompson R, Fragazy D. Attribution is more likely to be demonstrated in more natural contexts. Behavioural and Brain Sciences. 1998;21:124–126. [Google Scholar]
- Menzel EW. A group of young chimpanzees in a one-acre field. In: Schrier AM, Stollnitz F, editors. Behavior of nonhuman primates. San Diego, CA: Academic Press; 1974. pp. 83–153. [Google Scholar]
- Mulcahy NJ, Hedge V. Are great apes tested with an abject object-choice task? Animal Behaviour. 2012;83:313–321. [Google Scholar]
- Okamoto-Barth S, Call J, Tomasello M. Great apes’ understanding of other individuals’ line of sight. Psychological Science. 2007;18:462–468. doi: 10.1111/j.1467-9280.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- Oram MW, Wiener MC, Lestienne R, Richmond BJ. Stochastic nature of precisely timed spike patterns in visual system neuronal responses. Journal of Neurophysiology. 1999;81:3021–3033. doi: 10.1152/jn.1999.81.6.3021. [DOI] [PubMed] [Google Scholar]
- Oram MW, Hatsopoulos NG, Richmond BJ, Donoghue JP. Excess synchrony in motor cortical neurons provides redundant direction information with that from coarse temporal measures. Journal of Neurophysiology. 2001;86:1700–1716. doi: 10.1152/jn.2001.86.4.1700. [DOI] [PubMed] [Google Scholar]
- Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophysical Journal. 1967;7:391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povinelli DJ, Nelson KE, Boysen ST. Inferences about guessing and knowing by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1990;104:203–210. doi: 10.1037/0735-7036.104.3.203. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Eddy TJ. Factors influencing young chimpanzees’ (Pan troglodytes) recognition of attention. Journal of Comparative Psychology. 1996;110:336–345. doi: 10.1037/0735-7036.110.4.336. [DOI] [PubMed] [Google Scholar]
- Povinelli DJ, Bierschwale DT, Cech CG. Comprehension of seeing as a referential act in young children, but not juvenile chimpanzees. British Journal of Developmental Psychology. 1999;17:37–60. [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behavioural and Brain Sciences. 1978;1:515–526. [Google Scholar]
- Reaux JE, Theall LA, Povinelli DJ. A longitudinal investigation of chimpanzees’ understanding of visual perception. Child Development. 1999;70:275–290. [Google Scholar]
- Rohwer S, Ewald PW. The cost of dominance and advantage of subordination in a badge signalling system. Evolution. 1981;35:441–454. doi: 10.1111/j.1558-5646.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Schloegl C, Kotrschal K, Bugnyar T. Modifying the object-choice task: is the way you look important for ravens? Behavioural Processes. 2008b;77:61–65. doi: 10.1016/j.beproc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Shepherd SV, Platt ML. Spontaneous social orienting and gaze following in ring-tailed lemurs (Lemur catta) Animal Cognition. 2008;11:13–20. doi: 10.1007/s10071-007-0083-6. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Call J, Hare B. Five primate species follow the visual gaze of conspecifics. Animal Behaviour. 1998;55:1063–1069. doi: 10.1006/anbe.1997.0636. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Agnetta B. Chimpanzees, Pan troglodytes, follow gaze direction geometrically. Animal Behaviour. 1999;58:769–777. doi: 10.1006/anbe.1999.1192. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Lehmann H, Call J. Reliance on head versus eyes in the gaze following of great apes and human infants: The cooperative eye hypothesis. Journal of Human Evolution. 2007;52:314–320. doi: 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Hogrefe G-J, Perner J. Children’s understanding of informational access as a source of knowledge. Child Development. 1988;59:386–396. [Google Scholar]