Summary
Poly(ADP-ribose) polymerases (PARPs) catalyze poly(ADP-ribose) addition onto proteins, an important post-translational modification involved in transcription, DNA damage repair, and stem cell identity. Previous studies established the activation of PARP1 in response to DNA damage, but little is known about PARP1 regulation outside of DNA repair. We developed a new assay for measuring PARP activity in cell lysates, and found that the basal activity of PARP1 was highly variable across breast cancer cell lines, independent of DNA damage. Sucrose gradient fractionation demonstrated that PARP1 existed in at least three biochemically distinct states in both high and low activity lines. A newly discovered complex containing the NuA4 chromatin remodeling complex and PARP1 was responsible for high basal PARP1 activity, and NuA4 subunits were required for this activity. These findings present a new pathway for PARP1 activation and a direct link between PARP1 and chromatin remodeling outside of the DNA damage response.
Introduction
Poly(ADP-ribose) (PAR) is a reversible post-translational modification involved in multiple essential cellular processes including DNA damage, transcriptional control, and stem cell identity (Beneke, 2012; Chiou et al., 2013; Doege et al., 2012; Hassa and Hottiger, 2008; Ji and Tulin, 2010; Krishnakumar and Kraus, 2010a; Ogino et al., 2007; Tallis et al., 2013). Using NAD+ as a substrate, poly(ADP-ribose) polymerases (PARPs) polymerize ADP-ribose subunits onto acceptor proteins, forming large, negatively charged polymers of varying length (Schreiber et al., 2006; Tan et al., 2012). Polymers can be quickly hydrolyzed by poly(ADP-ribose) glycohydrolases (PARGs) leading to turnover of the NAD+ pool (Diefenbach and Burkle, 2005; Hassa and Hottiger, 2008). Covalent attachment of PAR to a protein (PARylation) can alter its function. PARP1, for example, loses its PARP activity upon auto-modification (Ferro and Olivera, 1982; Zahradka and Ebisuzaki, 1982). Alternatively, PAR can serve as a scaffolding molecule recruiting downstream PAR-binding effectors (Sousa et al., 2012). Seventeen putative PARPs have been identified in humans, based on sequence homology, (Schreiber et al., 2006), but not all possess PARP activity (Kleine et al., 2008). PARP1, localized primarily to the nucleus, is the most abundant family member in humans (Vyas et al., 2013; Wang et al., 2012) and has been mainly examined in the context of base excision repair (Sousa et al., 2012). Recently PARP1 was implicated in other DNA repair pathways as well as in pathways outside of DNA repair such as transcription (Ji and Tulin, 2013; Krishnakumar and Kraus, 2010b) and stem cell identity (Chiou et al., 2013; Doege et al., 2012; Ogino et al., 2007). The details of its involvement in any of these pathways remain poorly understood.
There is much interest in the use of PARP inhibitors as cancer therapeutics. At least six phase III trials are ongoing or being planned for PARP1 inhibitors (Garber, 2013). These trials focus mainly on targeting cancers with defects in homologous recombination (HR) in an effort to exploit the hypothesis that PARP1 inhibition is synthetically lethal with other DNA repair defects (Farmer et al., 2005; Javle and Curtin, 2011). However, the role of PARP1 in DNA damage does not fully explain the efficacy of PARP inhibitors (Audeh et al., 2010; Garnett et al., 2012; Lord and Ashworth, 2013). To better understand the utility of PARP inhibitors in the clinic, we must better understand the function and regulation of PARPs in cancer, especially PARP1, the common target of all the clinical candidates.
Despite their clinical as well as basic biological importance, fundamental questions about the regulation and cellular functions of PARPs remain unanswered. To explore potential rolls outside of the DNA damage response, we investigated basal PARP activity across breast cancer cell lines, and found, unexpectedly, large variation due to differences in basal PARP1 activation states and not in gene expression or protein abundance. Our findings provide a new pathway for PARP1 activation and suggest that PARP1 exists in different biochemical states both within a single cell line as well as between cell lines. Our findings further the basic understanding of PARP1 biochemistry and suggest new roles for PARP1 outside of the DNA damage response.
Results
Basal PARP1 activity varies strongly across breast cancer cell lines
To profile basal activation states of PARP, we measured PARP activity in cell lysates, in the absence of DNA damage, across a panel of breast cancer-derived cell lines. We used a bead based capture assay optimized for lysate measurements that allowed for better quantification of PAR levels than the standard immunoblot based assay. Our assay is complimentary to a recent mass spectrometry method quantifying steady-state PAR levels in cells or tissues (Martello et al., 2013). Lysates were prepared from cells grown under standard, non-stressed growth conditions. A PARG inhibitor, ADP-HPD, was added to the lysis buffer to prevent degradation of PAR during the assay, which was important since PAR degraded quickly in its absence. PAR was captured onto beads coated with an anti-PAR monoclonal antibody and detected using a tandem zinc-finger PAR binding domain from the protein APLF (Ahel et al., 2008). PAR, produced and purified in vitro (Tan et al., 2012), was used as a standard. Addition of a PARP inhibitor directly to the lysis buffer confirmed that the assay measures PAR accumulation in lysates, but not pre-formed PAR from cells, since most of the detectable PAR accumulated after lysate preparation (Figure S1). Thus the assay measures total PARP activity in lysate, not cellular PAR levels.
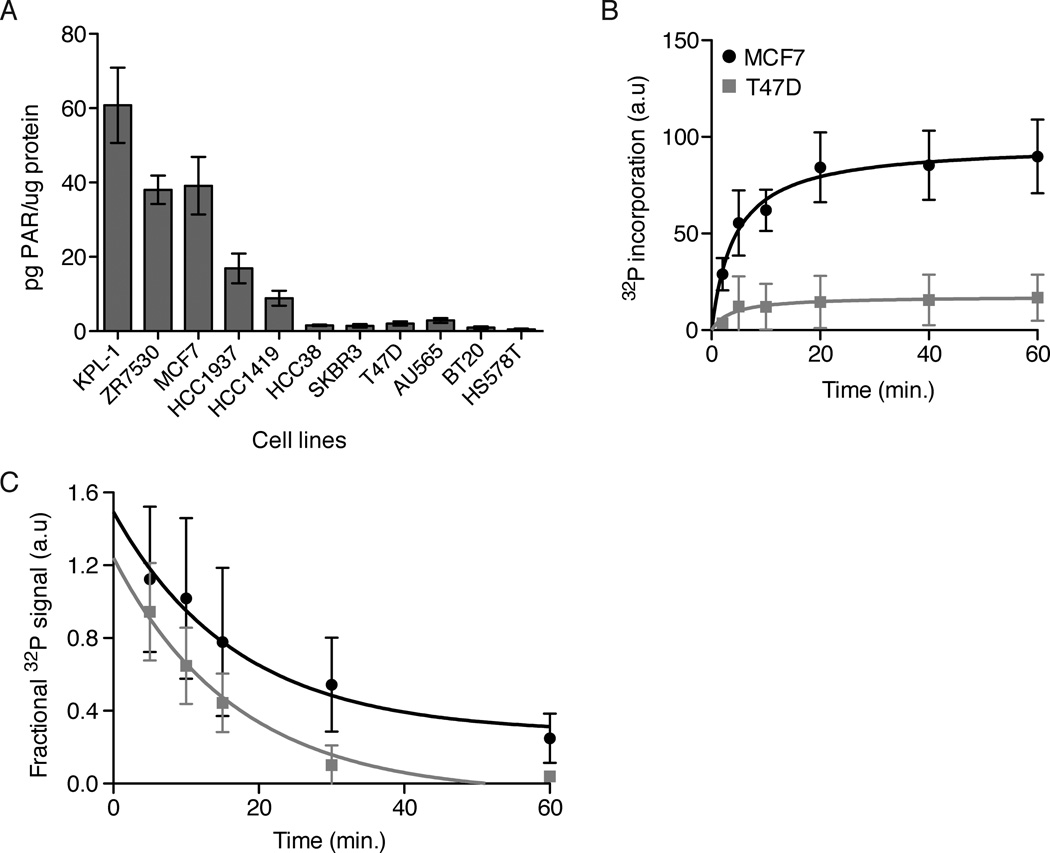
We found a surprising degree of variation in total PARP activity across 11 breast cancer cell line lysates, with up to 60-fold variation, using the new assay (Figure 1a). Differences in polymer levels were also seen by immunoblot for PAR, a standard assay in the field (Figure S1). We were unable to detect a PARP dependent signal by immunofluorescence (data not shown), confirming that out assay measures accumulation of PAR in the lysates and not basal levels. We further validated this result using a radioactive polymerization assay. Cell lysates from MCF7 (high activity) or T47D (low activity) cells were incubated with 32P-NAD+ in the presence of ADP-HPD, and the rate of PAR synthesis was measured using a filter-binding assay. Synthesis rates were much higher in MCF7 cell lysates (Figure 1b). The rate of PAR degradation was measured by adding preformed 32P labeled PAR to lysates in the presence of ABT-888 to block further synthesis. MCF7 and T47D cell lysates showed similar rates of PAR degradation (Figure 1c). We conclude that the basal activity of one or more PARPs is differentially regulated between MCF7 and T47D cells, while the rates of PAR degradation do not vary significantly in the two lines tested.
Figure 1. Basal PARP activity varies across cell lines.
A) As a proxy for PARP activity, PAR accumulation in lysates was measured across the breast cancer cell lines indicated. PAR levels were normalized by total protein in the lysates. Error bars show standard error and n ≥ 6. B) PARP activity in lysates was measured with 32P-NAD+ addition to lysates plus 1uM ADP-HPD. Data were fit with a onestep binding curve. Error bars show standard deviation and n=5. C) PAR degradation rates were measured by incubating pre-labeled 32P-PAR-PARP1 with lysates plus 10uM ABT-888. Data were fit with a one-step decay curve. Error bars show standard deviation and n=5. The decay rate is not significantly different for the two data sets, p=0.26 (calculated using Prism). See also Figure S1.
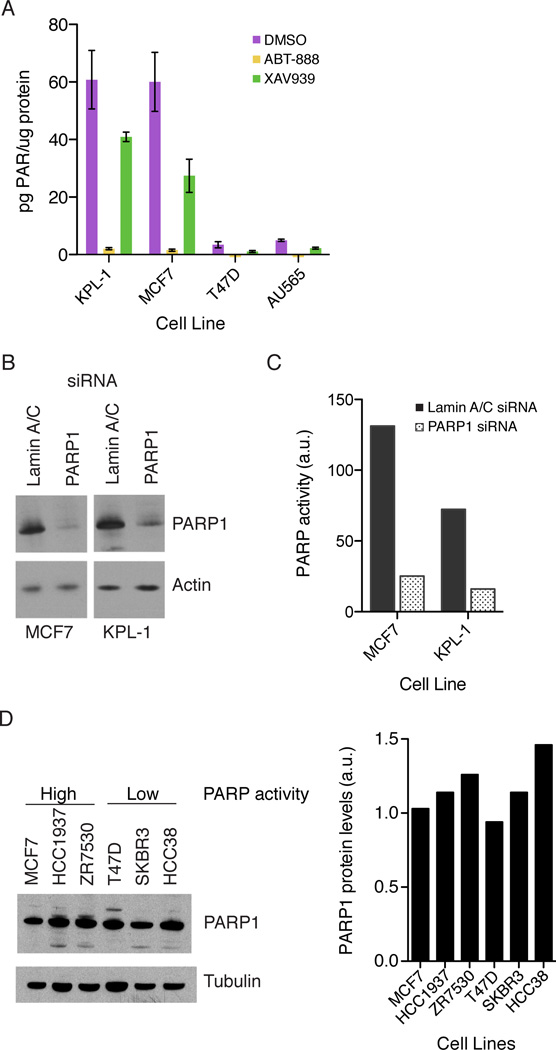
To determine which PARP family members are activated in MCF7 cells, we measured PARP activity in cell lysates from cells treated either with the PARP1-4 inhibitor ABT-888 or the PARP5a/b inhibitor XAV939 (Wahlberg et al., 2012). XAV939 also binds PARP1/2 but with approximately 10 fold lower affinity than to PARP5a/b (Huang et al., 2009; Wahlberg et al., 2012). Across the cell lines tested, PARP activity was reduced by > 97% in the presence of ABT-888, while XAV939 reduced PARP activity by 30–70% (Figure 2a). We further narrowed the possibilities with a PARP1/2 specific inhibitor, niraparib ((Jones et al., 2009), which reduced activity by ~95% (Figure S2a). Of PARP1 and 2, PARP1, due to its high abundance, was the most obvious candidate. Using siRNA, we knocked down PARP1 in two cell lines with high PARP activity, KPL-1 and MCF7. In both cell lines, PARP1 knockdown of 70–90% (Figure 2b) resulted in an approximately 80% loss in lysate PARP activity (Figure 2c). We confirmed by RT-PCR that PARP1 siRNA is specific to PARP1 and does not affect PARP2 RNA levels (Figure S2b). These data indicate that PARP1 is the primary enzyme responsible for the high basal PARP activity in the tested cell lines.
Figure 2. Differences in PARP activity result from PARP1 activation.
A) Cells were treated with DMSO (purple), 10uM ABT-888 (yellow) or 10uM XAV939 (green) for 2 hours and PAR accumulation in the lysates was measured. Total PAR is normalized by total protein in the lysates. Error bars show standard error with n = 6. B) PARP1 knock down in MCF7 and KPL-1 cells with Lamin A/C siRNA (Dharmacon) as a control. C) For PARP1 activity, control siRNA (solid bars) and PARP1 siRNA (open bars) lysates with equal amounts of total protein were incubated with 32P-NAD+ for 30 minutes. A representative experiment from 3 independent experiments is shown. D) PARP1 protein levels across the cell lines shown. Bands were quantified using ImageJ and PARP1 protein levels normalized by tubulin levels. See also Figure S2.
PARP1 activity could be regulated at either the mRNA or protein level. We compared PARP1 transcript levels across cell lines using published data (Neve et al., 2006). The small differences in transcript levels found seemed unlikely to account for the activity differences (data not shown). We then measured protein levels using immunoblots. Indeed, PARP1 protein levels were relatively constant across cell lines (Figure 2d), and the small differences observed did not correlate with differences in PARP1 activity. Instead, the results are consistent with the enzymatic activity of PARP1 being regulated through mechanisms other than transcription, translation, or degradation.
PARP1 activation is independent of DNA strand breaks
Based on the literature, our initial hypothesis was that PARP1 is basally activated in some cancer cell lines due to spontaneous DNA damage. To test this hypothesis, we measured DNA damage using phospho-histone H2Ax, which forms foci at the sights of DNA strand breaks, as a marker. Immunofluorescence measurements of phospho-H2Ax in fixed cells, grown under standard conditions in the absence of DNA damaging agents, showed faint staining in most lines, and much stronger staining following treatment with neocarzinostatin, an inducer of strand breaks. No significant correlation between basal phospho-H2Ax staining and basal PARP activity was found (Figure S3a–c). We also measured the levels of phospho-ATM, ATR, Chk1, and Chk2 as other markers for activation of the DNA damage repair pathways. We saw very little activation of these proteins in the absence of a DNA damaging agent (Figure S3d). We concluded that spontaneous DNA damage did not explain the differences in basal activation of PARP1 in these cell lines.
Another possible explanation is that DNA is released during lysate preparation, and its presence activated PARP1 in lysates. To test this, we blotted for histones in either the lysate supernatant or pellet. We found 70–80% of total PARP1 in the supernatant and no histone H4. Histone H4 was only found in the pellet (Figure S4a), indicating that the active PARP1 we were measuring was nucleoplasmic and not tightly bound to chromatin. It also suggested that no or very little chromatin was present in our lysate preparations. To further test for the presence of DNA, the DNA binding dye YOYO-1 was added to control lysates and lysates treated with DNase I. YOYO-1 fluoresces upon binding to nucleic acids and detects picograms of DNA. We observed weak signal in untreated lysates corresponding to 1–2pg of nucleic acid/ng of total protein (0.1% of the total protein). This signal was the same for both MCF7 and T47D lysates. The signal remained after DNase I treatment of the lysates (Figure S4b). We also ran pre-cleared and cleared lysate samples treated with RNase on agarose gels and stained for nucleic acids with SYBR Safe (Invitrogen) (Figure S4c). We saw no staining in the cleared lysate samples, indicating that DNA is removed from the lysates upon centrifugation. Based on the limits of detection in our YOYO-1 assay, these experiments show that there is less than 0.02pg of DNA/ng of protein in the lysate. The DNase resistant YOYO-1 signal may result from binding to RNA in the lysates. Even if a small amount of DNA is present, it appears to be the same for the two samples, and cannot account for the differences in PARP activity.
PARP1 is found in multiple biochemically distinct complexes
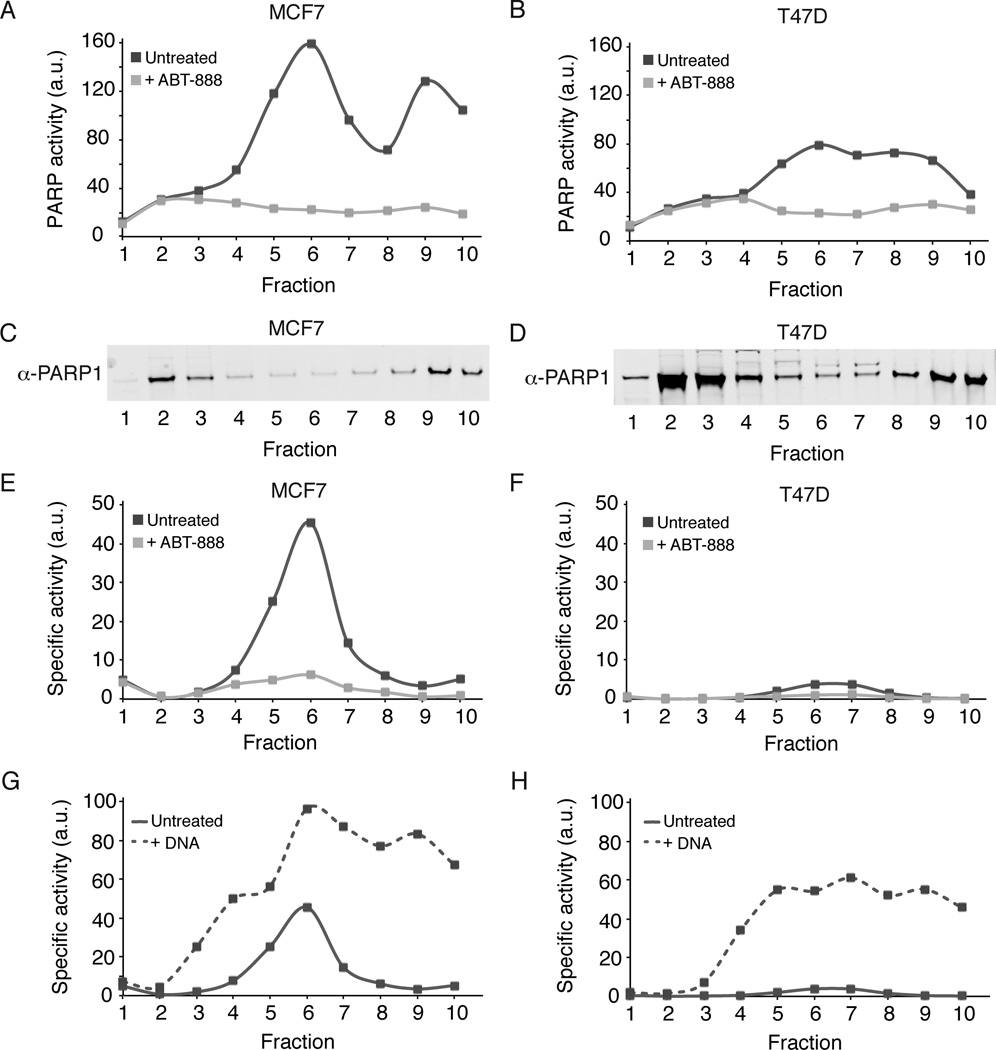
As an unbiased approach to characterizing cell line differences, we fractionated cell lysates using sucrose gradients and measured PARP activity and PARP1 protein levels across fractions. The PARP activity of each fraction was measured in the presence and absence of ABT-888 to confirm specificity of NAD+ incorporation (Figure 3a–b). The distribution of PARP1 protein between fractions was similar in both high and low activity lines (Figure 3c–d). Approximately 44% was found in a fraction whose mobility was that expected of un-complexed PARP1 sedimenting as a monomer or dimer. The sucrose gradient cannot distinguish between a monomer or a dimer, but previous studies have shown that PARP1 functions as a dimer (Mendoza-Alvarez and Alvarez-Gonzalez, 1993; Pion et al., 2005). ~10% of PARP1 protein was found in a ~700kD region, and ~35% in a high molecular weight region (>1MD). The distribution of PARP1 protein was similar in both cell lines.
Figure 3. PARP1 is found in multiple biochemical states.
MCF7 (A, C, E, G) and T47D (B, D, F, H) lysates were fractionated using 5–40% sucrose gradients. A-B) Aliquots from each fraction were incubated with 32P-NAD+ for 30 minutes in the absence (solid dark grey line) and presence (light grey line) of 10uM ABT-888. C-D) Aliquots from the same sucrose gradient fractions were used for PARP1 immunoblots. E-F) PARP1 activity from panels A-B was normalized by PARP1 protein levels to give specific activity. Representative data from two independent experiments is shown. G-H) Aliquots from each fraction were incubated in the absence (solid line) or presence (dashed line) of 0.02 mg/ml knicked DNA. Specific activity was determined as in panels E-F. See also Figure S4.
Basal PARP1 activity was detected by 32P-NAD+ polymerization. Activity in the uncomplexed PARP1 was undetectable. In MCF7 (high activity) lysates, PARP1 activity was strong in the 700kD and >1MD fractions. We compared activity to protein levels measured by immunoblot to estimate specific activity in arbitrary units, and found the highest specific activity in the ~700kD fraction (Figure 3e–f). This fraction was responsible for most (~75%) of the increased basal PARP1 activity in MCF7 cells as compared to T47D cells.
To determine if PARP1 activity could be increased, we added nicked DNA to each fraction and re-measured the PARP1 activity (Figure 3g–h). We found that even the most active PARP1 containing fractions could be further activated with the addition of nicked DNA. Both the 700kD and >1MD fractions were activated to approximately the same level of specific activity. The un-complexed fraction showed some increase in activity, but was the least activated in the presence of DNA. Taken together, the fractionation data indicated that PARP1 exists in at least three separate forms, each with a distinct basal activation state and potential for activation by nicked DNA. The difference in PARP activity between the two cell lines derived mainly from different basal activation states of the 700kD fraction.
PARP1 with high basal activity is in complex with NuA4
To find candidate binding partners, fractions 2+3, 6, and 9+10 from an MCF7 lysate sucrose gradient were immunoprecipitated with an anti-PARP1 antibody, and co-precipitating proteins were identified via tandem mass spectrometry of tryptic peptides (LC-MS/MS). Spectral counts of PARP1 peptides across the three fractions correlated well with the abundance of PARP1 protein measured by immunoblotting (Table 1, Table S1, and Figure 3c), showing quantitative recovery of PARP1 protein. We detected no significant binding partners in the un-complexed fraction. We were particularly interested in fraction 6 given its high basal PARP activity in MCF7 (high activity) cells as compared to T47D (low activity) cells. Enrichment analysis was performed on the top 50 proteins immunoprecipitated from fraction 6 using the DAVID bioinformatics platform (Huang et al., 2008). The top enrichment group consisted of eight members of the 16-member NuA4 chromatin-modifying complex; this enrichment was also obvious from visual inspection of the data. Further analysis of fraction 6 LC-MS/MS data identified 14 subunits of the NuA4 complex that were highly enriched for fraction 6 when compared to fractions 2+3 and fractions 9+10 (Table 1, Table S1).
Table 1.
Active PARP1 co-immunoprecipitates with components of the NuA4 complex
| Sucrose Gradient | SILAC | |||
|---|---|---|---|---|
| Spectral counts per fraction | ln(MCF7/T47D ratio) | |||
| Gene Name | 2+3 | 6 | 9+10 | Average |
| PARP1 | 147 | 27 | 47 | 0 |
| TRRAP | 93 | 6 | ||
| EP400 | 6 | 69 | 4 | 0.63 ± 0.34 |
| RUVBL1 | 31 | 7 | 0.64 ± 0.074 | |
| RUVBL2 | 28 | 10 | ||
| EPC1 | 19 | 1.9 ± 0.17 | ||
| ACTL6A | 13 | 4 | 1.1 ± 0.092 | |
| DMAP1 | 13 | 1 | 1.2 ± 0.15 | |
| BRD8 | 10 | 1 | 0.93 ± 0.42 | |
| YEATS4 | 7 | 2 | ||
| ING3 | 5 | |||
| VPS72 | 5 | 1 | 0.81 ± 0.25 | |
| KAT5 | 3 | 1.8 ± 0.18 | ||
| MORF4L1 | 2 | 1 | 1.1 ± 0.16 | |
| MORF4L2 | 2 | |||
PARP1 was immunoprecipitated from the indicated sucrose gradient fractions (Sucrose Gradient) or total lysates (SILAC). For SILAC, one cell line was grown with heavy amino acids to facilitate quantification of the relative abundance of peptides between the two cell lysates. Spectral counts, a measure of relative abundance, are the number of MS2 spectra for a given protein. SILAC ratios, relative protein abundance between the two cell lines, were normalized to the ratio for PARP1, the natural log (ln) of each ratio was taken and the results of two biological repeats were averaged. The ln(ratios) for NuA4 proteins found in both repeats are shown. See also Table S1.
To test the hypothesis that NuA4 binding correlated with PARP1 activity, relative levels of PARP1 binding partners were compared between MCF7 total lysates and T47D total lysates using SILAC (stable isotope labeling by amino acids in culture) in combination with LC-MS/MS. PARP1 was immunoprecipitated from MCF7 and T47D lysates after growing one cell line in heavy amino acids, allowing for the relative quantification of proteins identified in both cell lines. Nine subunits of the NuA4 complex were enriched in MCF7 PARP1 immunoprecipitations from two biological repeats (Table 1). Thus, more NuA4 is in complex with PARP1 in the high activity line. These data are consistent with a hypothesis that binding to NuA4 activates PARP1 in the high activity line.
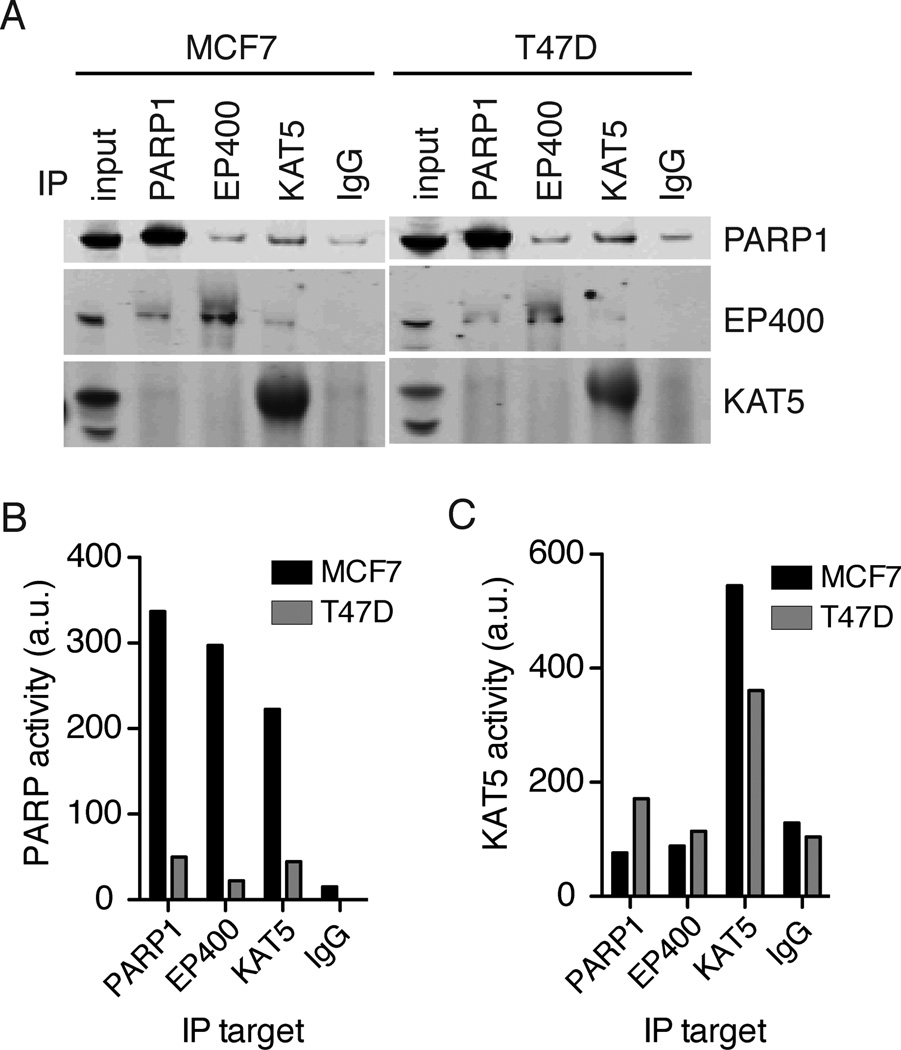
To re-test the PARP1-NuA4 interaction, reciprocal immunoprecipitations were performed by immunoprecipitating either the EP400 or KAT5(Tip60) subunits of the NuA4 complex and measuring PARP1 by immunoblot (Figure 4a). PARP1 co-immunoprecipitated with both NuA4 subunits in both MCF7 and T47D cell lysates. A small amount of PARP1 non-specifically bound to IgG, but levels of PARP1 in the EP400 and KAT5 immunoprecipitations were higher than in the IgG control. In the reverse, EP400 coimmunoprecipitated with both PARP1 and KAT5. A small amount of KAT5 may coimmunoprecipitate with PARP1 and EP400, but it was difficult to measure due to high background in the immunoblot. We next measured the PARP activity of separately immunoprecipitated PARP1, EP400, and KAT5 (Figure 4b). The highest PARP1 activity was observed for immunoprecipitated PARP1 from MCF7 cell lysates. Immunoprecipitated EP400 and KAT5 from MCF7 cell lysates also demonstrated high PARP1 activity, confirming that active PARP1 is in complex with NuA4. Immunoprecipitated EP400 and KAT5 from T47D cell lysates also showed PARP1 activity, but at much lower levels than MCF7. This was consistent with the lysate measurements and the sucrose gradient data where a small peak of activity was seen at ~700kD for T47D. These data indicated that NuA4 binds either active (from MCF7 cells) or inactive (from T47D cells) PARP1.
Figure 4. PARP1 forms two biochemically distinct complexes with NuA4.
A) Immunoprecipitations were performed from 1mg of total protein with the indicated antibodies. Immunoprecipitated proteins were probed for PARP1, EP400 or KAT5 by immunoblotting. The input sample contains 15ug of total protein. B) PARP1 activity, via 32P-NAD+ incorporation, and C) KAT5 activity, via in vitro acetylation of histone H4, were measured in each immunoprecipitated complex from MCF7 cell lysates (dark bars) and T47D cell lysates (light bars). Representative data from three independent experiments is shown. See also Figure S5.
To test if active KAT5 was part of the active PARP1-NuA4 complex, we measured acetyltransferase activity in the PARP1, EP400 and KAT5 immunoprecipitations. Neither immunoprecipitated PARP1 or EP400 from MCF7 had acetyltransferase activity above the IgG control. Immunoprecipitated PARP1 and EP400 from T47D cells showed a small amount of activity. In both cell lines, immunoprecipitated KAT5 displayed high acetyltransferase activity (Figure 4c). These data indicated that KAT5 activity was not required for PARP1 activation in MCF7 cells.
To test if the NuA4-PARP1 complex is specific to cancer cells we repeated the immunoprecipitation and activity experiments in RPE cells. RPE cells are immortalized, non-cancer cells. They had low basal PARP activity, comparable to T47D cells (Figure S5a). In RPE cell lysates, PARP1 with low activity immunoprecipitated with both EP400 and KAT5, and the acetytransferase activity of the PARP1 and EP400 immunoprecipitations was at the limit of detection (Figure S5b–d). Together with our previous results, we conclude that the PARP1-NuA4 complex is conserved across cancer and non-cancer cell lines, however, the level of PARP1 activity within the complex differs between cell lines with higher activity observed in some cancer cell lines such as MCF7.
Functional consequences of the PARP1-NuA4 interaction
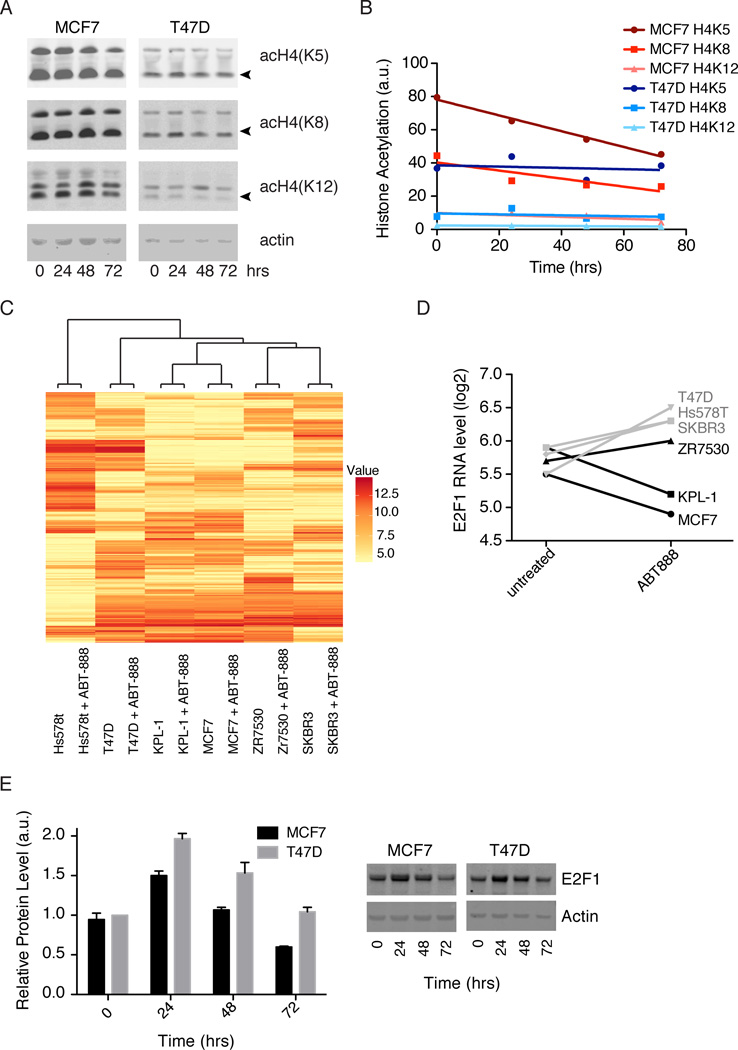
While acetylation was not essential for PARP1 activation, PARP1 activity may affect histone acetylation by KAT5. We measured the acetylation levels of a known target of KAT5, histone H4 (Doyon and Côté, 2004). We treated cells for up to 72 hours with the PARP1/2 inhibitor, niraparib, and then blotted for H4 acetylation at the K5, K8, or K12 positions (Figure 5a). No differences were seen for T47D. For MCF7, a small but significant decrease in acetylation of K5 was seen over 72 hours (Figure 5b).
Figure 5. Effects of PARP1 activity on histone acetylation and transcription.
A) Histone acetylation at the indicated positions in MCF7 and T47D cells was measured by immunoblot after 0, 24, 48, and 72 hours of treatment with 10uM niraparib. DMSO was used for the 0 time point. B) Intensities of acetylated histones (marked with arrows in A) were quantified in ImageJ and normalized by actin. Normalized intensities were plotted and fit with straight lines. The slope of MCF7 H4K5, −0.48 ± 0.03, is significantly different from zero with R2 = 0.99 and p = .005. The other fits are not significantly different from zero with p > 0.1. Fitting done in Prism. C) Hierarchical clustering of the 500 genes with the largest standard deviation across all 12 samples. Cell lines were treated for 48 hours with DMSO or 10uM ABT-888 before transcript profiling. Values shown are log2(expression level) D) Log2(expression levels) for E2F1 ± ABT-888 in cell lines with low PARP activity (grey) and cell lines with high PARP activity (black). E) E2F1 protein levels, determined by immunoblot, after 0, 24, 48 or 72 hours of 10uM niraparib treatment. Intensities of E2F1 were quantified in ImageJ and normalized by actin. See also Figure S6 and Table S2.
NuA4 is also involved in gene regulation (Ginsburg et al., 2009; Yamada, 2012), and we hypothesized that high basal PARP1 activity of a PARP1-NuA4 complex affects transcription. We therefore measured global transcript levels via microarray in the presence and absence of PARP inhibitors. First, we compared MCF7 cells treated for six hours with DMSO, ABT-888 or olaparib, two chemically distinct PARP1 inhibitors. Remarkably, given the abundance and high basal activity of PARP1 in MCF7 cells, we found no significant differences in transcript levels (Figure S6), suggesting that the PARP1-NuA4 complex is not a direct regulator of transcription. To test for later transcriptional effects, we repeated the microarray experiment with cells treated with either ABT-888 or DMSO for 48 hours. We compared six cell lines, three with high PARP activity (MCF7, KPL-1, and ZR7530) and three with low PARP activity (T47D, SKBR3, and Hs578T). Cell line specific differences dominated the differences in transcription, and from a global perspective PARP inhibitor treatment caused little change in transcript levels as compared to cell line differences. Unsupervised clustering placed drug-treated and untreated next to each other for all lines (Figure 5c). These data showed that PARP1 inhibitors were not strong regulators of global transcription. For a closer look at drug-induced changes, we binned the data into two groups, high PARP activity and low PARP activity, took the mean of each group, and ranked the genes by the difference of the means (Table S2). Our top hit, E2F1, is a transcription factor involved in cell cycle progression that has been implicated in apoptosis and as a tumor suppressor (Wang et al., 1999). E2F1 showed an increase in transcript levels in low PARP activity cell lines and a decrease in high activity cell lines following drug treatment (Figure 5d). We next examined E2F1 protein levels in MCF7 and T47D cells after 24, 48 and 72 hours of niraparib treatment (Figure 5e). In both cell lines, there was an initial increase in E2F1 protein followed by a gradual decline. T47D cells (low PARP activity) showed consistently higher levels of E2F1 than MCF7 cells, consistent with RNA levels. At 72 hours of drug treatment, E2F1 protein level in T47D cells was equivalent to the untreated level, whereas, the level in MCF7 cells was lower than the untreated level. We saw similar results in two additional cell lines, KPL-1 (high activity) and SKBR3 (low activity) (Figure S6). These data indicate a role for PARP activity in regulating both RNA and protein levels for E2F1 and the magnitude of this regulation is different between the high and low PARP activity cell lines.
Chromatin remodeling subunits of NuA4 are required for high basal PARP1 activity
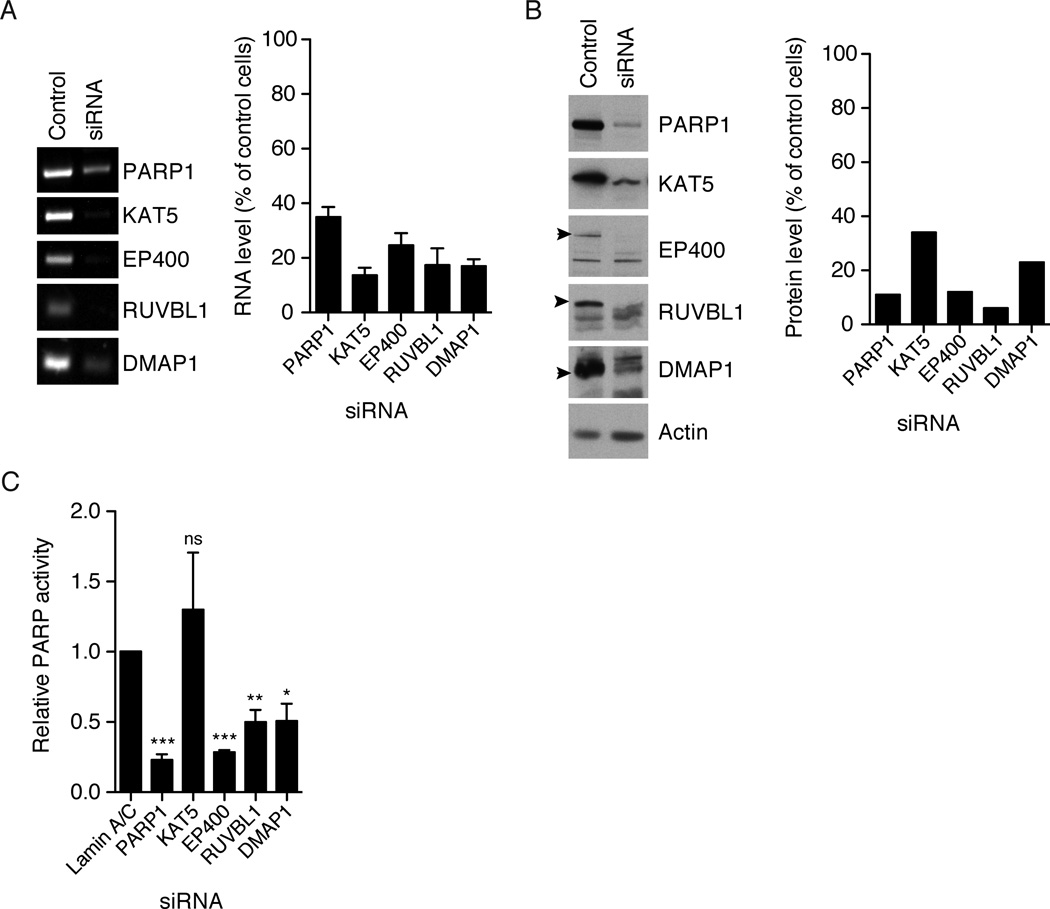
To test the hypothesis that NuA4 is required for activation of PARP1, we knocked down multiple subunits of NuA4 in MCF7 cells and measured basal PARP1 activity in lysates. Using siRNA, we knocked down the EP400, KAT5, RUVBL1 and DMAP1 subunits of NuA4. Knockdown of EP400 showed the most dramatic effect on PARP1 activity (Figure 6) reducing PARP1 activity to a similar extent as knockdown of PARP1 itself. RUVBL1 and DMAP1 knockdown also showed a reproducible decrease in PARP1 activity although not to the same low levels as EP400 knockdown (Figure 6). This might be explained by partial knockdown of the siRNA target. Knockdown of KAT5 had no significant effect on basal PARP1 activity in MCF7 cells. Loss of PARP activity in the EP400, RUVBL1 and DMAP1 knockdowns could be explained by a concomitant loss of PARP1. We examined PARP1 protein levels in all knockdowns and found that NuA4 knockdowns did not significantly affect PARP1 levels (data not shown). Lack of an effect of KAT5 knockdown is consistent with the immunoprecipitation data suggesting that acetyltransferase activity is not required to activate PARP1. Rather, subunits of NuA4 involved in chromatin remodeling, EP400, RUVBL1 and DMAP1 (Yamada, 2012), are required for high basal PARP1 activity in MCF7 cells.
Figure 6. NuA4 components are required for PARP1 activity.
Proteins were knocked down by siRNA in MCF7 cells. A) Representative image of RT-PCR on control and siRNA treated cells with quantification of the results using ImageJ for background subtraction and band intensity measurements. Error bars show standard error and n=3. B) Protein levels in control and siRNA treated cells as measured by immunoblot, and quantification of the shown immunoblot using ImageJ. C) PARP1 activity measured by incubating lysates with 32P-NAD+ for 30 minutes. Samples were normalized by total protein concentrations and reported as fraction of activity as compared to the lamin A/C siRNA control. Error bars show standard error with n=4. A t-test (Prism) comparing each siRNA to the lamin A/C control, p values are *** = < 0.001, ** = 0.001–0.01, * = 0.01–0.05, ns = > 0.05.
Discussion
We found that PARP1 in cell lysate was distributed between at least three complexes differing in native molecular weight and interacting proteins. A low molecular weight fraction, which comprised almost half of the total PARP1, moved on sucrose gradients consistent with the expected mass of un-complexed PARP1. This fraction was basally inactive by our means of detection, and interestingly, was not activated by nicked DNA, unlike bacteria-expressed PARP1. We speculated that this un-complexed fraction was somehow modified to prevent its activation by nicked DNA. Two larger molecular weight pools of PARP1 (approximately 700kD and >1MD) contained most of the basal and DNAinducible activity. The >1MD fraction is the least well understood. It contained ~35% of the PARP1 polypeptide, had low levels of basal specific activity but was highly inducible by nicked DNA. Mass spectrometry has not yet provided a consistent profile of PARP1 interacting proteins in this fraction, which is clearly worthy of further study. We concentrated our efforts on characterizing the fraction around 700kD, which had the highest basal activity per unit protein, the largest difference between cell lines, and appeared to consist in part of a defined complex between PARP1 and NuA4. The components of the NuA4 complex have been previously identified in HeLa cells, but PARP1 was not identified (Doyon et al., 2004). Based on our results, PARP1 appears to be a dynamic member of the NuA4 complex, whose interaction was likely disrupted by the higher salt concentrations used in the HeLa cell purification.
Our primary focus was to understand the 10–60 fold differences in basal PARP1 activity found between breast cancer cell lines. Surprisingly, the distribution of PARP1 protein on sucrose gradients was similar between a high activity cell line, MCF7, and a low activity cell line, T47D. The intermediate sized fraction, where PARP1 is complexed to NuA4, was hard to detect by PARP1 immunoblot alone, but immunoprecipitation data showed that this complex was present in both lines. Our data so far showed that the distribution of PARP1 between complexes was similar between cell lines, and that the difference was restricted to strong activation of PARP1 in MCF7 cells and minimal activation (or possibly repression of activity) in T47D. This differential activation was strongest in the NuA4 complex. This complex was not limited to cancer cells and could be found in a non-cancer cell line, RPE, where it had biochemical characteristics similar to the low activity complex found in T47D cells.
Having identified the PARP1-NuA4 interaction, we began to investigate the mechanism of PARP1 activation. Through siRNA studies, we found that chromatin remodeling components of NuA4 were required for the activation of PARP1. However, because NuA4 bound PARP1 in both high and low activity cell lines, binding alone, though necessary, was not sufficient for activation. In Drosophila, KAT5 is required for activation of PARP1 upon heat shock (Petesch and Lis, 2011). Our experiments indicated that KAT5 activity was not necessary for PARP1 activation. The data, suggested that levels of NuA4, in particular EP400, may determine PARP1 activity. We examined transcript levels of EP400 across cell lines and found no correlation with PARP activity (Figure S7). Also, we see no significant differences in EP400 protein levels between MCF7 and T47D cell lysates, and the amount of PARP1 that co-immunoprecipitates with EP400 is similar between the two cell lines. Instead of the amount or stoichiometry of the complex determining PARP1 activity, other enzymatic activities of NuA4 such as the ATPase activity of EP400 may be required, or NuA4 may facilitate the interaction of PARP1 with other activating factors. We are continuing to explore the mechanistic details of PARP1 activation in these cell lines.
Though KAT5 activity is not required for PARP1 activation, PARP1 may influence the histone acetylation functions of KAT5. We saw a small effect of PARP1 activity on the levels of acetylated histone H4(K5) in a high PARP activity cell line, and no effect in a low activity cell line, suggesting that PARP1 may play a role in regulating histone acetylation. While our data is consistent with PARP1 regulating histone acetylation through the NuA4 complex, we cannot rule out the involvement of other acetyltransferases. H4(K5) acetylation is not specific to NuA4, and other acetyltranserases could modify this position.
Both PARP1 and NuA4 have reported roles in transcription (Doyon and Côté, 2004; Ji and Tulin, 2010; Krishnakumar and Kraus, 2010a). We found no significant effect on transcription after 6 hours of PARP inhibitor treatment, indicating that the PARP1-NuA4 complex does not a directly regulate transcription. Surprisingly, we also found no strong global effect on transcription after 48 hours of PARP inhibitor treatment. One possibility is that high PARP1 activity primes cells to respond to other signals, and the effects of PARP1 inhibition on transcription would be larger in the presence of another stimulus, as was seen in the case of prostate cancer cells treated with testosterone (Schiewer et al., 2012). By looking across multiple cell lines, we found PARP dependent regulation of E2F1. This is consistent with a previous study showing PARP1 regulation of E2F1 transcription in fibroblasts (Simbulan-Rosenthal et al., 2003), and we confirmed regulation at the protein level. E2F1 is an important transcription factor with potential roles as a tumor suppressor, and this regulatory interaction may be one reason why some cancers upregulate PARP1 activity.
We undertook a comparative analysis of breast cancer cell lines in part to understand differential responses to PARP1 inhibitors in patients. We have uncovered new biochemistry of PARP1 suggesting that PARP1 inhibitors may work through mechanisms other than the currently proposed mechanisms of DNA repair. Cell lines with both high and low basal PARP1 activity were similarly unaffected by PARP1 inhibitors in culture, at least using standard cytotoxicity assays (not shown). However, high basal PARP activity might increase sensitivity to other drugs or combinations with PARP inhibitors.
Finally, our results highlight the importance of looking at differences in enzymatic activity when comparing cell lines. Many large-scale studies focus on transcript level differences between cancer cell lines, but as our study highlights, potentially important activity differences could be overlooked. Our study of basal differences in PARP1 activation highlights a new functional interaction for PARP1 with a chromatin-remodeling complex and paves the way for further elucidating non-stress induced roles of PARP1 in the nucleus and the impact of those roles on cancer cell biology.
Materials & Methods
Cell culture and lysis
All cell lines (ATCC) except KPL-1 and RPE were grown in RPMI (Mediatech/Corning) supplemented with 10% fetal bovine serum (FBS, Gibco). KPL-1 and RPE were grown in DMEM/F12 (Mediatech/Corning) supplemented with 10% FBS. For SILAC, cells were grown in RPMI without lysine or arginine (Caisson Labs) supplemented with 10% FBS and either light (12C, 14N) or heavy (13C, 15N) arginine and lysine (Sigma or Pierce). Cells harvested via trypsinization were washed with PBS and frozen at −80°C. Upon thawing, cells were lysed in lysis buffer (50mM Tris pH 8, 150mM NaCl, 1% NP-40) for 30 minutes on ice. Cell debris was pelleted at 4°C and 21,000×g for 30 minutes. Lysate protein concentration was determined with the Bio-Rad DC protein assay. For acetylation, E2F1, and DNA damage marker immnoblots, cells were lysed directly in the tissue culture dish with 1× SDS loading buffer. Lysate was collected and sonicated before boiling and loading the gel.
Antibodies
For immunoblotting the following antibodies were used: anti-PARP1 (Tulip Biolabs, 1051), anti-histone H4 and anti-acetyl-histone H4 (Lys5, 8 or 12) (Cell Signaling, 8346), monoclonal anti-tubulin clone DM1A (Sigma), monoclonal anti-actin clone AC-74 (Sigma), anti-phospho ATM, ATR, CHK1, and CHK2 (Cell Signaling, 9947), anti-DMAP1 (Bethyl, A300-218A), anti-Tip60(KAT5) (Imgenex, IMG-6313A), anti-rabbit Dylight 800 (Thermo), anti-mouse Dylight 680 (Thermo), anti-chicken IgY Dylight 800 (Thermo), anti-chicken IgY HRP (Abcam), anti-mouse HRP (GE Healthcare), and anti-rabbit HRP (GE Healthcare). Proteins were visualized using the ECL 2 kit (Thermo) for HRP detection or the Odyssey (Licor) for far-red (Dylight) fluorescent detection. For immunoprecipitation the following antibodies were used: anti-PARP (Cell Signaling, 46D11), anti-Tip60(KAT5) (Calbiochem, DR1041), and anti-EP400 (Novus, NB200-210). Anti-phospho histone H2Ax (Ser139) (Upstate, 05-636) was used for immunofluorescence followed by anti-mouse Alexa488 (Invitrogen.
Protein Purification
The tandem zinc finger domain of human APLF (residues 376–450, APLF-PBD) was cloned into pET28a (Invitrogen) and expressed in E. coli strain BL21(DE3) Rosetta pLysS (Novagen). Upon induction with IPTG, 500uM ZnSO4 was added to the media. Protein was expressed overnight at 16°C. Cells were resuspended in 50mM Tris pH 7.4, 1M NaCl, 150uM ZnSO4, 10% Glycerol, 1% Tween, 5mM β-mercaptoethanol, and complete protease inhibitor cocktail (Roche) and lysed using a cell disruptor. Cell debris was pelleted for 30 minutes at 138,000×g, and the supernatant was incubated with Ni-NTA resin (Qiagen) at 4°C for 2 hours. The nickel resin was washed with 20mM Tris pH 7.4, 1M NaCl, 150uM ZnSO4, 5mM β-mercaptoethanol, and 20mM imidazole and eluted with 20mM Tris pH 7.4, 200mM NaCl, 150uM ZnSO4, 5mM β-mercaptoethanol, and 300mM imidazole. APLF-PBD was then gel filtered on a Sephacryl S-100 column (GE Healthcare) using 50mM Tris pH 7.4, 200mM NaCl, 100uM ZnSO4, and 5mM β-mercaptoethanol.
PARP1 and the catalytic fragment of PARG were purified as previously described (Tan et al., 2012).
PAR detection in cellular lysates
Monoclonal PAR antibody 10H (Tulip Biolabs) was conjugated to carboxylated MagPlex beads (Luminex/Bio-rad). Briefly, 5×106 beads/ml were washed and resuspended in coupling buffer (50mM monobasic sodium phosphate, pH 5.0). 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM, Oakwood Products) was added to a final concentration of 5mg/ml and incubated with the beads at room temperature for 20 minutes. Beads were washed in coupling buffer, resuspended with 8ug antibody in coupling buffer, and incubated for two hours at room temperature. Conjugated beads (MagPlex-10H) were washed and stored at 4°C in PBS with 0.02% Tween, 0.1% BSA and 0.05% azide.
To biotinylate APLF-PBD, 1.7mg/ml purified protein was incubated with 0.5mg/ml sulfo-NHS-LC-biotin (Invitrogen) for one hour at room temperature followed by one hour at 4°C. The reaction was quenched with 100mM glycine.
For PAR detection all lysates contained 1uM ADP-HPD (Axxora, EMD). All dilutions and washes were done with PBS + 1% BSA. MagPlex-10H beads (50 beads/ul) and lysate (50–200ng total protein/ul) were incubated together overnight at 4°C. Beads were then washed, resuspended in 3ng/ul biotin-APLF-PBD, and incubated for two hours at room temperature. Next, beads were washed, resuspended in 2ng/ul conjugated streptavidin-RPhycoerythrin (Thermo), and incubated at room temperature for 30 minutes. Finally, beads were washed, resuspended to a final concentration of 50 beads/ul, and analyzed on the FlexMap3D (Luminex). Free PAR, purified as previously described (Tan et al., 2012), was used as a standard for quantification.
32P PARP and PARG activity assays
For PARP activity, lysates (0.5 – 1 mg total protein/ml) were incubated with 5µCi 32P-NAD+ (Perkin-Elmer, 800Ci/mmol 5mCi/ml) and 1uM ADP-HPD in PBS or lysis buffer at 30°C. Reactions were quenched by adding trichloroacetic acid (TCA, Sigma) to a final concentration of 10%. Quenched reactions were filtered through nitrocellulose, pre-wet in PBS, and washed with 1% TCA. 32P incorporation was quantified using a phosphor storage screen and scanner (Bio-Rad). 10uM ABT-888 (Selleck Chemicals) was added as a control for PARP activity.
For PARG activity assays 32P-labeled PAR-PARP1 was made through an automodification reaction as described previously (Tan et al., 2012). Lysate (0.5 – 1 mg total protein/ml) and 10uM ABT-888 were added to the 32P-PAR-PARP1 in PBS. The reactions were quenched and analyzed as for the 32P PARP activity assay.
Immunoprecipitation
Whole cell lysates or sucrose gradient fractions containing a protease inhibitor cocktail (Roche) were incubated with antibody for two hours at 4°C. Pre-washed affiprep Protein A beads (BioRad) were added and incubated for one hour at 4°C with the antibody/antigen complex. Beads were washed with 50mM Tris pH 8.0, 50mM NaCl, 1mM EGTA, 1mM MgCl2. SILAC samples were washed with 50mM Tris pH 8.0, 150mM NaCl, 1mM EGTA, 1mM MgCl2. For SDS-PAGE analysis, proteins were eluted from the beads by boiling in sample buffer, and immunoblots were performed using the Licor Quick Western Dectection Kit. Activity assays were performed directly on beads.
Sucrose gradient fractionation
Sucrose gradients (5–40% w/w) were poured as step gradients of five equal volume steps in 50mM Tris pH 8.0, 50mM NaCl, 1mM EGTA, and 1mM MgCl2 and allowed to diffuse overnight into continuous gradients. Gradients were spun at 4°C and 237,000×g for 4 hours in an SW55Ti rotor (Beckman). Gradients were fractionated from the top by pipetting. A standard containing thyroglobulin, catalase, aldolase and BSA was run in parallel.
LC-MS/MS analysis
PARP1 immunoprecipitation samples from SILAC experiments were reduced in 20mM DTT (Sigma), alkylated with 100mM iodoacetamide (Thermo), and separated on SDS-PAGE. Each lane was cut into 6 pieces and digested with trypsin (Promega). Sucrose gradient PARP1 immunoprecipitation samples were eluted from beads with 8M urea in 50mM Tris pH 8.0, reduced in 20mM DTT, alkylated with 40mM iodoacetamide, quenched with 10mM DTT, diluted to less than 1M urea with 50mM Tris pH 8.0, and digested with trypsin (Promega). Samples were run on a Thermo Fisher Q Exactive coupled with Exigent LC system (AB Sciex, Framingham, MA) over a one hour gradient. Peptide identification and quantification was performed with MaxQuant v1.3.05 (Cox et al., 2009).
KAT5 in vitro activity assay
Beads from KAT5 IPs were mixed with 0.2mg/ml histone H4 peptide (SignalChem) and 10uM acetyl-CoA (EMD Millipore), incubated for 30 minutes at 30°C, and spotted onto nitrocellulose. The membrane was blocked and probed with anti-acetyl H4(K8) (Cell Signaling). A far-red fluorescent secondary antibody was used for imaging on the Odyssey (Licor). Background subtraction and quantification was done with the ImageJ MBF plugin suite (http://rsb.info.nih.gov/ij/plugins/mbf/index.html).
RNAi
Cells were transfected with siRNA using either Lipofectamine2000 or RNAiMax (Invitrogen) according to the manufacturer’s protocol. RNAi was done with a double transfection protocol over 7 days total. Cells were allowed to recover for three days after the first transfection, retransfected, and then allowed to grow for an additional three days. All siRNAs were ordered from Thermo and the following siRNAs were used; PARP1 ONTARGETplus (GAUUUCAUCUGGUGUGAUA or CCAAUAGGCUUAAUCCUGU), EP400 siGENOME siRNA SMARTpool, KAT5 siGENOME siRNA SMARTpool, RUVBL1 siGENOME siRNA SMARTpool, DMAP1 siGENOME siRNA SMARTpool.
RNAi efficiency was tested via RT-PCR or immunoblot. Total RNA was extracted from cells with the Qiagen RNeasy kit and RT-PCR was done with the One-step RT-PCR kit (Qiagen) using the following primer pairs;
PARP1: GCCCTAAAGGCTCAGAACGA and AGCTTCCCGAGAGTCAGGAT,
EP400: TCCCAGCAGCAACCATTTCA and ATTCCTCCTTGTGCGGTCG,
KAT5: AGGGGGAGATAATCGAGGGC and CACCTTCCGTTTCGTTGAGC,
RUVBL1: TGGTTTTCCACGCTGGTTTT and CTCCTTTATTCGCAGCCCAATG,
DMAP1: CAAGGCCCCCAAAAAGAAGC and GCTTTCTCGAATTGGGCGTG.
PARP2: AGGTCATGGGCCAGCAAAAG and ACGGAGTCCAAAGTCATGCG
Transcriptional profiling
Cells were treated with DMSO, olaparib, or ABT-888 (Selleck Chemicals) for 6 or 48 hours and harvested via trypsinization. Total RNA was extracted using the Qiagen RNeasy Plus kit. Samples were submitted to the Boston Children’s Hospital Microarray Core Facility for transcriptional profiling using Illumina HT-12 beadchips. Initial data analysis including normalization and background subtraction was performed with the Illumina GenomeStudio software package. The quantile-background subtracted data was used for further analysis using the R platform.
Supplementary Material
Highlights.
PARP1 activity varies strongly across cell lines, independent of DNA strand breaks
PARP1 exists in at least three biochemically distinct states
Basal activity of a PARP1-NuA4 complex accounts for differences across cell lines
The NuA4 complex is required for the high basal activity of PARP1
Acknowledgements
We would especially like to thank Cyril Benes for helpful discussions and comments throughout the project and for providing the KPL-1 cell line. For technical help, we thank Saima Ahmed, Kristina Hempel, Nurhan Ozlu, Sujeong Kim, Nathan Moerke, Mirra Chung, and Edwin Tan. We also thank Sujeong Kim, and Lingyin Li for comments on the manuscript. Illumina expression analysis was performed in the BCH IDDRC Molecular Genetic Core that is supported by National Institutes of Health award NIH-P30-HD 18655. K.A.K was a Fayez Sarofim Fellow of the Damon Runyon Cancer Research Foundation (DRG-2053-10). This work was supported by 1 K99 CA175176-01 (K.A.K) and 5 P01 CA139980-04 (T.J.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession numbers
Transcript profiling data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession numbers GSE58844 and GSE56400.
Author Contributions
K.A.K., J.S., and T.J.M designed the study. K.A.K performed the experiments and analyzed the data. R.J. analyzed the transcriptional data. K.A.K. and T.J.M wrote the manuscript.
None of the authors have any conflicts of interest related to the work in this study.
Supplemental Data Inventory
Figure S1: PAR quantification assay measures PARP activity in lysates, related to Figure 1
Figure S2: PARP1 is the main contributor to PARP activity in lysates, related to Figure 2
Figure S3: Basal PARP activation does not correlate with DNA damage, related to results section “PARP1 activation is independent of DNA strand breaks”
Figure S4: DNA is not a major component of lysate preparations, related to Figure 3
Figure S5: The PARP1-NuA4 complex is also found in a non-cancer cell line, related to Figure 4
Figure S6: Transcriptional response of cells to PARP inhibition, related to Figure 5
Figure S7: EP400 transcript levels do not correlate with PARP1 activity, related to discussion section
Table S1: Top 50 proteins in the sucrose gradient fraction 6 PARP1 immunoprecipitation, related to Table 1
Table S2: Changes in transcript expression levels after PARP inhibition in three high PARP activity and three low PARP activity cell lines, related to Figure 5
References
- Ahel I, Ahel D, Matsusaka T, Clark A, Pines J, Boulton S, West S. Poly (ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451:81–85. doi: 10.1038/nature06420. [DOI] [PubMed] [Google Scholar]
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib inpatients with. The Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- Beneke S. Regulation of chromatin structure by poly(ADP-ribosyl)ation. Front. Genet. 2012;3:1–16. doi: 10.3389/fgene.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon P, Côté J. Precise deposition of histone H2A.Z in chromatin for genome expression and maintenance. BBA - Gene Regul. Mech. 2012;1819:290–302. doi: 10.1016/j.bbagrm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Chiou SH, Jiang BH, Yu YL, Chou SJ, Tsai PH, Chang WC, Chen LK, Chen LH, Chien Y, Chiou GY. Poly(ADP-ribose) polymerase 1 regulates nuclear reprogramming and promotes iPSC generation without c-Myc. J. Exp. Med. 2013;210:85–98. doi: 10.1084/jem.20121044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Matic I, Hilger M, Nagaraj N, Selbach M, Olsen JV, Mann M. A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nat. Protoc. 2009;4:698–705. doi: 10.1038/nprot.2009.36. [DOI] [PubMed] [Google Scholar]
- Diefenbach J, Burkle A. Introduction to poly(ADP-ribose) metabolism. Cell. Mol. Life Sci. 2005;62:721–730. doi: 10.1007/s00018-004-4503-3. [DOI] [PubMed] [Google Scholar]
- Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R, Guarnieri P, Bhagat G, Vanti WB, Shih A, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon Y, Côté J. The highly conserved and multifunctional NuA4 HAT complex. Curr. Opin. Genetics Dev. 2004;14:147–154. doi: 10.1016/j.gde.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Doyon Y, Selleck W, Lane WS, Tan S, Côté J. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol. Cell. Biol. 2004;24:1884–1896. doi: 10.1128/MCB.24.5.1884-1896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Fazzio TG, Huff JT, Panning B. Chromatin regulation Tip(60)s the balance in embryonic stem cell self-renewal. Cell Cycle. 2008;7:3302–3306. doi: 10.4161/cc.7.21.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro AM, Olivera BM. Poly(ADP-ribosylation) in vitro. Reaction parameters and enzyme mechanism. J. Biol. Chem. 1982;257:7808–7813. [PubMed] [Google Scholar]
- Garber K. PARP inhibitors bounce back. Nat. Cell Biol. 2013;12:725–727. doi: 10.1038/nrd4147. [DOI] [PubMed] [Google Scholar]
- Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, Greninger P, Thompson IR, Luo X, Soares J, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg DS, Govind CK, Hinnebusch AG. NuA4 lysine acetyltransferase Esa1 is targeted to coding regions and stimulates transcription elongation with Gcn5. Mol. Cell. Biol. 2009;29:6473–6487. doi: 10.1128/MCB.01033-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci. 2008;13:3046–3082. doi: 10.2741/2909. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang S, Mishina Y, Liu S, Cheung A, Stegmeier F, Michaud G, Charlat O, Wiellette E, Zhang Y, Wiessner S. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Javle M, Curtin NJ. The role of PARP in DNA repair and its therapeutic exploitation. Br. J. Cancer. 2011;105:1114–1122. doi: 10.1038/bjc.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Tulin A. The roles of PARP1 in gene control and cell differentiation. Curr. Opin. Genetics Dev. 2010;20:512–518. doi: 10.1016/j.gde.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Tulin A. Post-Transcriptional Regulation by Poly(ADP-ribosyl)ation of the RNA-Binding Proteins. Int. J. Mol. Sci. 2013;14:16168–16183. doi: 10.3390/ijms140816168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Altamura S, Boueres J, Ferrigno F, Fonsi M, Giomini C, Lamartina S, Monteagudo E, Ontoria JM, Orsale MV, et al. Discovery of 2-{4-[(3S)-piperidin- 3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADPribose) polymerase (PARP) inhibitor efficacious in BRCA-1 and-2 mutant tumors. J. Med. Chem. 2009;52:7170–7185. doi: 10.1021/jm901188v. [DOI] [PubMed] [Google Scholar]
- Kleine H, Poreba E, Lesniewicz K, Hassa PO, Hottiger MO, Litchfield DW, Shilton BH, Lüscher B. Substrate-Assisted Catalysis by PARP10 Limits Its Activity to Mono-ADP-Ribosylation. Mol. Cell. 2008;32:57–69. doi: 10.1016/j.molcel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Krishnakumar R, Kraus W. The PARP Side of the Nucleus: Molecular Actions, Physiological Outcomes, and Clinical Targets. Mol. Cell. 2010a;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar R, Kraus WL. PARP-1 Regulates Chromatin Structure and Transcription through a KDM5B-Dependent Pathway. Mol. Cell. 2010b;39:736–749. doi: 10.1016/j.molcel.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 2013;19:1381–1388. doi: 10.1038/nm.3369. [DOI] [PubMed] [Google Scholar]
- Martello R, Mangerich A, Sass S, Dedon PC, Bürkle A. Quantification of Cellular Poly(ADP-ribosyl)ation by Stable Isotope Dilution Mass Spectrometry Reveals Tissue- and Drug-Dependent Stress Response Dynamics. ACS Chem. Biol. 2013;8:1567–1575. doi: 10.1021/cb400170b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Alvarez H, Alvarez-Gonzalez R. Poly (ADP-ribose) polymerase is a catalytic dimer and the automodification reaction is intermolecular. J. Biol. Chem. 1993;268:22575–22580. [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe J-P, Tong F, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H, Nozaki T, Gunji A, Maeda M, Suzuki H, Ohta T, Murakami Y, Nakagama H, Sugimura T, Masutani M. Loss of Parp-1 affects gene expression profile in a genome-wide manner in ES cells and liver cells. BMC Genomics. 2007;8:41. doi: 10.1186/1471-2164-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petesch SJ, Lis JT. Activator-Induced Spread of Poly(ADP-Ribose) Polymerase Promotes Nucleosome Loss at Hsp70. Mol. Cell. 2011;45:64–74. doi: 10.1016/j.molcel.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion E, Ullmann GM, Amé J-C, Gérard D, De Murcia G, Bombarda E. DNA-induced dimerization of poly(ADP-ribose) polymerase-1 triggers its activation. Biochemistry. 2005;44:14670–14681. doi: 10.1021/bi050755o. [DOI] [PubMed] [Google Scholar]
- Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, Dean JL, Liu F, Planck JL, Ravindranathan P, Chinnaiyan AM, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame J, De Murcia G. Poly (ADP-ribose): novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- Simbulan-Rosenthal CM, Rosenthal DS, Luo R, Samara R, Espinoza LA, Hassa PO, Hottiger MO, Smulson ME. PARP-1 binds E2F-1 independently of its DNA binding and catalytic domains, and acts as a novel coactivator of E2F-1-mediated transcription during re-entry of quiescent cells into S phase. Oncogene. 2003;22:8460–8471. doi: 10.1038/sj.onc.1206897. [DOI] [PubMed] [Google Scholar]
- Sousa FG, Matuo R, Soares DG, Escargueil AE, Henriques JAP, Larsen AK, Saffi J. PARPs and the DNA damage response. Carcinogenesis. 2012;33:1433–1440. doi: 10.1093/carcin/bgs132. [DOI] [PubMed] [Google Scholar]
- Tallis M, Morra R, Barkauskaite E, Ahel I. Poly(ADP-ribosyl)ation in regulation of chromatin structure and the DNA damage response. Chromosoma. 2013;123:79–90. doi: 10.1007/s00412-013-0442-9. [DOI] [PubMed] [Google Scholar]
- Tan ES, Krukenberg KA, Mitchison TJ. Large-scale preparation and characterization of poly(ADP-ribose) and defined length polymers. Anal. Biochem. 2012;428:126–136. doi: 10.1016/j.ab.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas S, Chesarone-Cataldo M, Todorova T, Huang Y-H, Chang P. A systemic analysis of the PARP protein family identifies new functions critical for cell physiology. Nat. Commun. 2013;4:1–13. doi: 10.1038/ncomms3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg E, Karlberg T, Kouznetsova E, Markova N, Macchiarulo A, Thorsell A-G, Pol E, Frostell AS, Ekblad T, uuml DON. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat. Biotech. 2012;30:283–288. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]
- Wang M, Weiss M, Simonovic M, Haertinger G, Schrimpf SP, Hengartner MO, Mering von C. PaxDb, a Database of Protein Abundance Averages Across All Three Domains of Life. Mol. Cell. Prot. 2012;11:492–500. doi: 10.1074/mcp.O111.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Dong L, Saville B, Safe S. Transcriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol. Endo. 1999;13:1373–1387. doi: 10.1210/mend.13.8.0323. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ayrapetov MK, Xu C, Gursoy-Yuzugullu O, Hu Y, Price BD. Histone H2A.Z Controls a Critical Chromatin Remodeling Step Required for DNA Double-Strand Break Repair. Mol. Cell. 2012;48:723–733. doi: 10.1016/j.molcel.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada HY. Ettarh R, editor. Human Tip60 (NuA4) Complex and Cancer. Colorectal Cancer Biology - From Genes to Tumor. 2012 (InTech) [Google Scholar]
- Zahradka P, Ebisuzaki K. A shuttle mechanism for DNA-protein interactions. The regulation of poly(ADP-ribose) polymerase. Eur. J. Biochem. 1982;127:579–585. [PubMed] [Google Scholar]
- Zobeck KL, Buckley MS, Zipfel WR, Lis JT. Recruitment timing and dynamics of transcription factors at the Hsp70 loci in living cells. Mol. Cell. 2010;40:965–975. doi: 10.1016/j.molcel.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.