Figure 1.
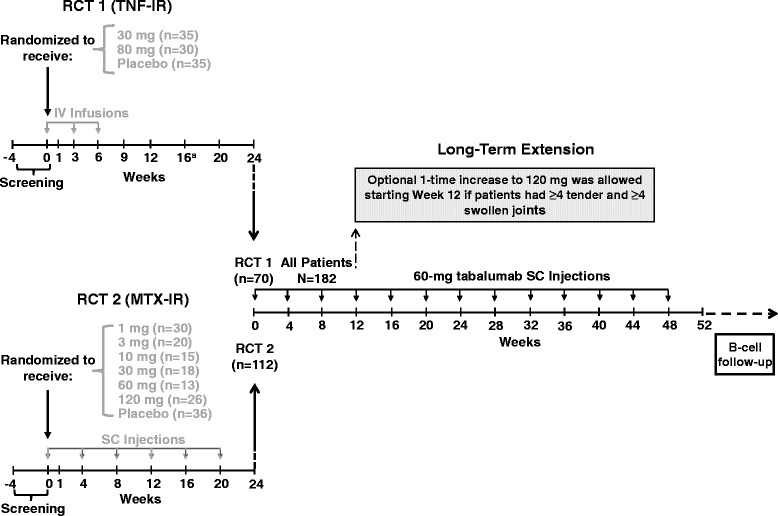
Randomized controlled trial and open-label extension study designs. aNonresponders (<20% improvement in tender or swollen joint counts in 28 joints) were eligible to receive an additional 30-minute infusion of 80-mg tabalumab as a rescue dose at week 16. IV, Intravenous; MTX-IR, Methotrexate inadequate responders; n, Number of patients per treatment arm; N, Total number of patients enrolled; RCT, Randomized controlled trial; SC, Subcutaneous; TNF-IR, Tumor necrosis factor inadequate responders.
