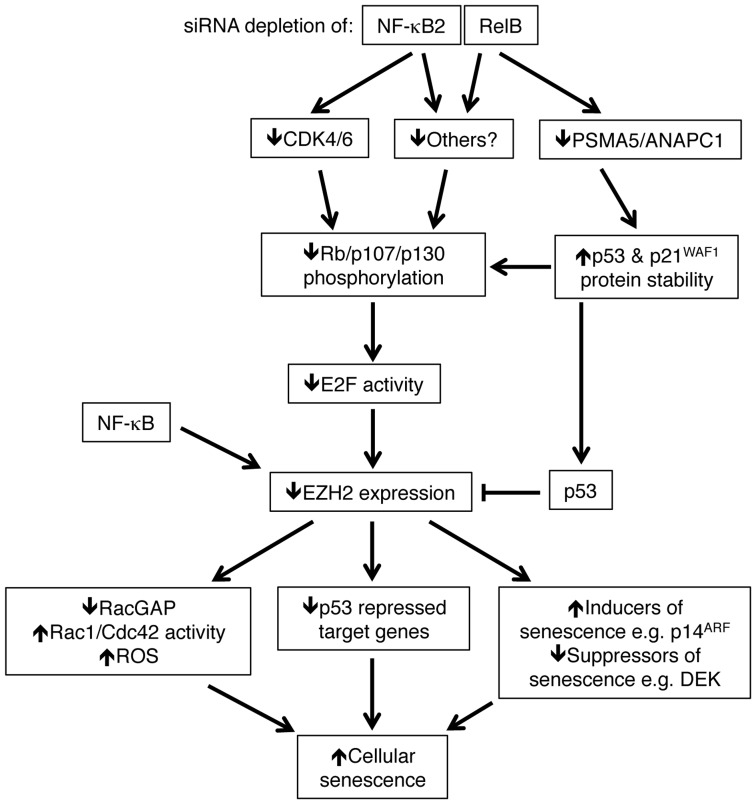
Figure 11. Summary of the results identified in this manuscript through which NF-κB2 and RelB regulate p53 dependent cellular senescence in primary human NHD fibroblasts.
Depletion of NF-κB2 and RelB leads to a decrease in Rb phosphorylation. Unphosphorylated Rb represses E2F transcriptional activity and consequently inhibits EZH2 expression (which is negatively regulated by p53). However, this occurs through distinct pathways. Depletion of NF-κB2 leads to down regulation of CDK4 and CDK6, which are known to phosphorylate Rb directly. RelB depletion leads to an increase in p53 and p21 protein stability as a consequence of loss of expression of genes such as PSMA5 and ANAPC1. Other gene targets may be involved in these processes. NF-κB subunits also bind directly to the EZH2 promoter and this may also contribute towards its regulation. As a consequence of this pathway, down regulation of EZH2 results in numerous changes in gene expression, including p53 dependent repression of a number of gene targets. These include inhibition of RACGAP1 expression, resulting in Rac1/Cdc42 dependent induction of reactive oxygen species (ROS). Together with other changes, such as suppression of DEK and ultimately induction of p14ARF and p16INK4a, the ultimate consequence of NF-κB2 and RelB siRNA depletion and subsequent loss of EZH2 expression is p53 dependent cell senescence. Note, RACGAP1 activity as well as various inducers/suppressors or senescence may also be regulated by p53. Many of these genes may also be direct targets of NF-κB. Not shown is that oxidative stress is required to drive the basal level p53 activity seen in these cells.