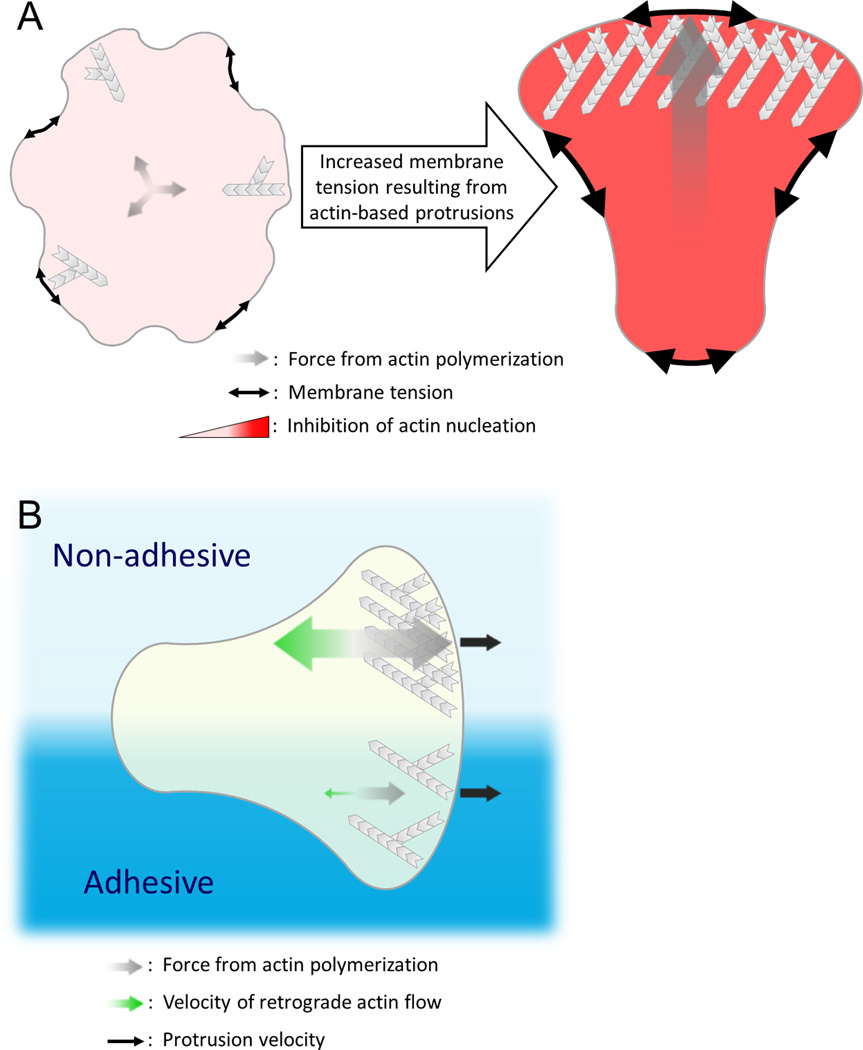
Figure 2. Actin assembly reciprocally interacts with mechanical forces to regulate protrusion number and speed.
A) In the presence of uniform chemoattractant, cells form a single leading edge through competition between nascent protrusions. This competition occurs through long-range inhibition that is communicated by changes in membrane tension. Actin polymerization at each protrusion exerts force on the plasma membrane (grey arrows), rapidly increasing membrane tension (black double-arrows) throughout the cell. Increased membrane tension globally suppresses the formation of new sites of polarized actin assembly through inhibition of upstream signals such as Rac activation and WRC recruitment; however, assembly still occurs at the initial site since positive feedback components are already present at sufficiently high levels to overcome this inhibition. This system ensures that a single dominant leading edge emerges [66], but it is not yet clear how changes in tension are translated to changes in signaling. B) Dendritic cells in a confined environment maintain a steady rate of forward protrusion, independent of surface adhesiveness. To compensate for reduced traction on non-adhesive surfaces and the subsequent increase in the rate of retrograde flow of the actin network (green arrows), cells increase the rate of actin polymerization directed toward the leading edge (grey arrows) to produce the same protrusion velocity of the plasma membrane (black arrows). Even a single cell that finds itself overlapping surfaces with differing levels of adhesiveness is able to maintain distinct subcellular domains with different rates of actin assembly to produce the same net force across the leading edge and maintain persistence despite the adhesive differences. These data suggest that the speed of cell protrusion is not set by the amount of actin assembly but rather depends on how fast the plasma membrane is released into the protrusions [52]. How this is regulated is unknown.