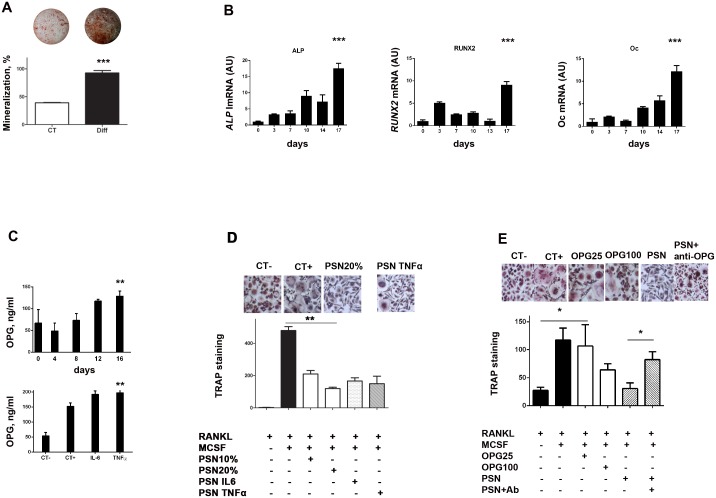
Figure 4.
(A) Human primary pericytes underwent osteoblastic differentiation when cultured in an osteogenic media, as shown by Alizarin red staining. Representative images are above corresponding bars. (B) PCR experiments confirmed that during this process, pericytes increasingly expressed the ALP (p<0.0001), Runx2 (p<0.0001) and osteocalcin (Oc) (p<0.0001) characteristic markers of osteoblasts. (C) CD14+ cells were cultured during 14 days with standard media (CT−) or with addition of M-CSF and RANKL (CT+), after which TRAP staining was realized and giant multinucleated cells counted. Addition of pericytes supernatant (PSN) to the medium (in the proportion of 10% and 20%) dose-dependently inhibited the osteoclastic differentiation. A similar result was obtained when IL-6 and TNF-α pericyte-conditioned supernatant were used. Representative images are displayed above the bars. (D) Elisa experiments showed that pericytes expressed increasing amounts of OPG along their osteoblastic differentiation (upper graph) but also under inflammatory stimulation by IL-6 and TNF-α (lower graph). (E) CD14+ cells were cultured to undergo osteoclastic differentiation as in (C). Addition of OPG (25 ng/ml and 100 ng/ml), inhibited the formation of multinucleated cells. Addtion of PSN produced similar effect. Finally, addition of an antibody directed against OPG (PSN + anti-OPG) significantly reversed this inhibition. All experiments were realized three times in triplicate. CT-: media with RANKL, CT+: media with RANKL and MCSF.