Table 2.
Reaction of α-Ketoamides 1 with α,β-Unsaturated Aldehydes 2
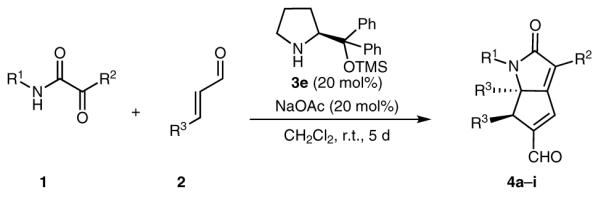
| |||||
|---|---|---|---|---|---|
|
| |||||
| Producta | R1 | R2 | R3 | Yieldb (%) | eec,d (%) |
| 4a | Ph | Ph | Ph | 63 | 97 |
| 4b | Ph | Ph | 4-MeOC6H4 | 51 | 89 (91) |
| 4c | Ph | Ph | 4-ClC6H4 | 34 | 85 (95) |
| 4d | Ph | Ph | 2,3-(OCH2O)C6H3 | 56 | 84 (87) |
| 4e | 4-MeOC6H4 | Ph | Ph | 66 | 92 (91) |
| 4f | 3-ClC6H4 | Ph | Ph | 69 | 91 (95) |
| 4g | 4-O2NC6H4 | Ph | Ph | 58 | 95 |
| 4h | Ph | 2-MeC6H4 | Ph | 70 | 88 |
| 4i | Ph | 4-ClC6H4 | Ph | 71 | 95 |
Reaction conditions: 0.3-mmol scale using α-ketoamide 1 (1 equiv), α,β-unsaturated aldehyde 2 (2 equiv), NaOAc (20 mol%), 3e (20 mol%), CH2Cl2 (1 mL), r.t. All the products were obtained as a single diastereomer.
Yield of isolated 4a–i.
Determined by HPLC on a chiral stationary phase.
Values in brackets correspond to the results obtained with the catalyst (R)-3e. For HPLC determination of the enantiomeric excess, the products 4b–i were transformed into the corresponding α,β-unsaturated ethyl ester.
