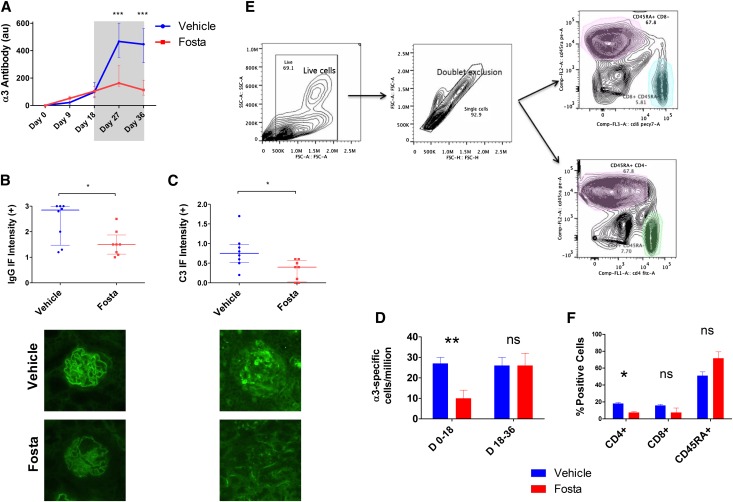
Figure 6.
SYK inhibition terminates pathogenic autoantibody production in experimental anti-GBM disease. (A) Circulating anti-GBM antibody levels in fostamatinib- (Fosta; red plots) and vehicle-treated (blue) animals during the 18-day treatment-free period (unshaded) and the 18-day treatment period (shaded) show cessation of autoantibody production after introduction of fostamatinib treatment. (B) At day 36, fostamatinib-treated animals had less deposited anti-GBM antibody, which was detected by direct IF. Lower panel photomicrographs show representative linear IF for deposited anti-GBM antibodies in fostamatinib- and vehicle-treated animals at day 36. Original magnification, ×400. (C) Fostamatinib-treated animals also had less complement C3 deposition within glomeruli, suggesting reduced activation of the classic complement pathway secondary to decreased deposition of antibody. Lower panel photomicrographs show representative IF for C3 in glomeruli of vehicle- and fostamatinib-treated rats. (D) α3-Specific splenic B cells were enumerated by B-cell ELISpot assay. Fostamatinib treatment from day 0 to day 18 inhibited the generation of α3-specific splenic B cells (measured at day 18). Fostamatinib treatment from day 18 to day 36 did not affect the overall number of α3-specific splenic B cells at day 36. Replicate results (×8) from one of three representative biologic replicated experiments are shown. (E) Gating strategy for flow cytometric analysis of splenocyte subsets. (F) Fostamatinib treatment for 18 days did not significantly affect the survival of CD45RA- or CD8-positive splenocytes, although there was a reduction in CD4-positive cells. Animals: n=8/group, except flow cytometric analysis (n=4/group). Data are reported as median per group±interquartile range. *P<0.05; **P<0.01; ***P<0.001.