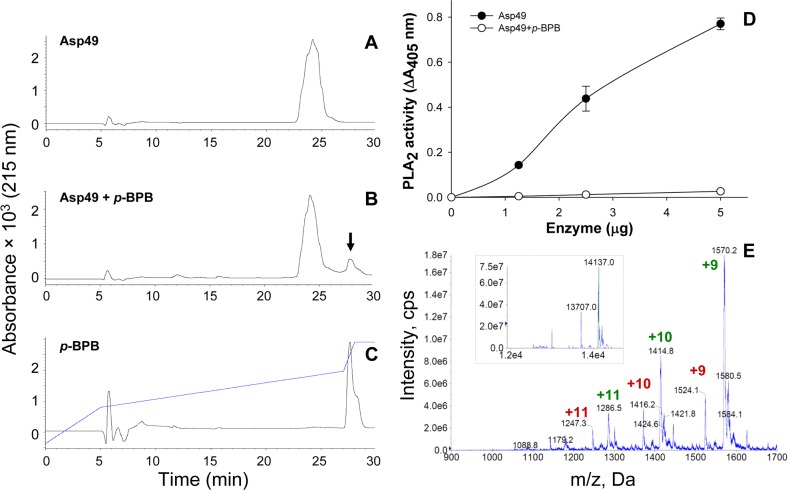
Figure 4. Modification of the Asp49 phospholipase A2 by p-bromophenacyl bromide (p-BPB).
(A) unmodified enzyme control; (B) p-BPB-treated enzyme; and (C) p-BPB reagent control. The three samples were separated by RP-HPLC in a semi-preparative C8 column as in Fig. 1B. (D) Comparison of the phospholipase A2 (PLA2) activities of the control Asp49 enzyme and the p-BPB-treated enzyme on the synthetic monodisperse substrate 4-nitro-3-octanoyloxybenzoic acid. Each point represents mean ± SD of three replicates. (E) Nano-electrospray mass spectrometry confirmation of the covalent incorporation of a single molecule of p-BPB in the modified Asp49 myotoxin. The observed isotope-averaged masses of the modified Asp49 preparation show an increase of 195 ± 3 Da in comparison to the masses of untreated proteins (Fig. 2D).