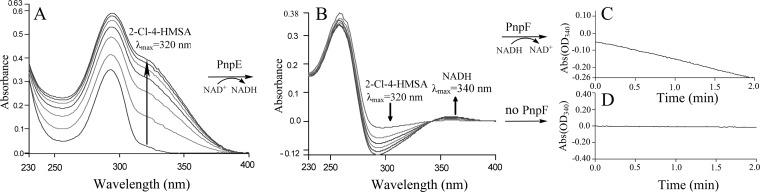
FIG 7.
Assays of PnpCD, PnpE, and PnpF enzyme activities by sequential catalytic reactions with CHQ as the starting substrate. (A) Spectral changes during rapid oxidation of CHQ by purified His6-PnpCD. Sample and reference cuvettes contained 20 mM phosphate buffer (pH 7.4), 0.04 mM Fe2+, and 5 μg purified His6-PnpCD in a 0.5-ml volume. The reaction was initiated by the addition of 0.1 mM CHQ. Spectra were recorded every minute after the addition of CHQ. (B) Enzyme activity assay of PnpE. Following the complete oxidation of CHQ to 2-chloro-4-hydroxymuconic semialdehyde by PnpCD, 50 μM NAD+ was added to the sample cuvette from the reaction in panel A and then the contents were divided between two cuvettes (sample and reference) for assay of PnpE activity. Spectra were recorded every minute after the addition of 20 μg purified H6-PnpE. (C, D) His6-PnpF enzyme activity assays. Following the complete conversion of 2-chloro-4-hydroxymuconic semialdehyde to 2-chloromaleylacetate, 50 μM NADH was added to the sample cuvette from the reaction in panel B and then the contents were divided between two cuvettes (sample and reference) for assay of PnpF activity. To initiate the assay, 20 μg purified H6-PnpF (C) or buffer without H6-PnpF (D) was added to the sample cuvette.