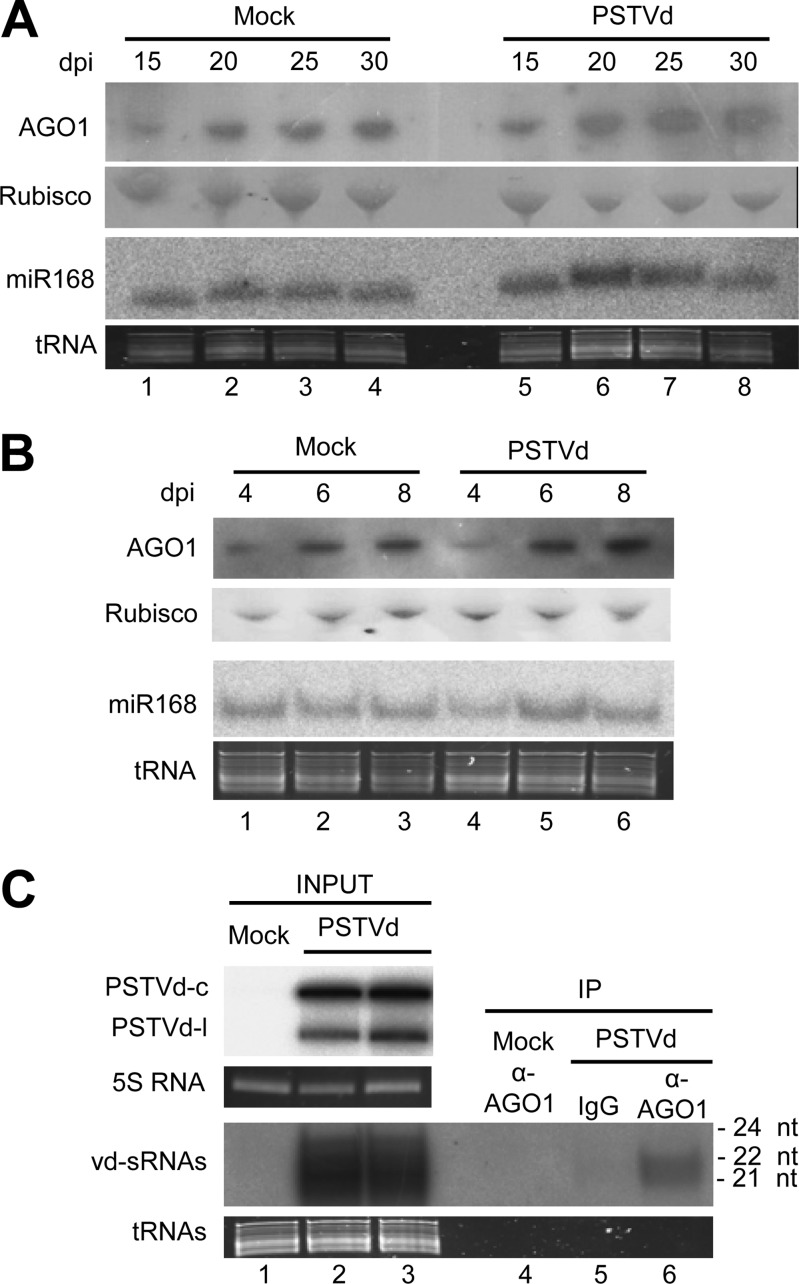
FIG 1.
(A and B) PSTVd infection of N. benthamiana has no significant effect on AGO1 or miR168 accumulation in the upper, noninoculated leaves (A) or in the agroinfiltrated leaves (B). Western blot analyses were performed with a rabbit polyclonal antibody against the N-terminal region of AGO1 from N. benthamiana and a goat anti-rabbit secondary antibody conjugated to horseradish peroxidase. Total proteins were separated by PAGE in 4 to 12% gels, and equal loading was assessed by the intensity of the large subunit of RubisCO after staining with Ponceau S. Northern blot hybridizations were carried out with a 5′-radiolabeled oligodeoxyribonucleotide complementary to miR168. Total RNAs were separated by denaturing PAGE in 17% gels, and equal loading was assessed by the intensity of tRNA after staining with ethidium bromide. (A) Samples were collected at 15 (lanes 1 and 5), 20 (lanes 2 and 6), 25 (lanes 3 and 7), and 30 (lanes 4 and 8) days p.i. (dpi). (B) Samples were collected at 4 (lanes 1 and 4), 6 (lanes 2 and 5), and 8 (lanes 3 to 6) days p.i. (C) Endogenous AGO1 loads vd-sRNAs during PSTVd infection of N. benthamiana. Aliquots of total sRNA (INPUT) and of the sRNA fraction immunoprecipitated with a rabbit polyclonal antibody against the N-terminal region of AGO1 from N. benthamiana (α-AGO1) (IP) were separated by denaturing PAGE in 17% gels and revealed by Northern blot hybridization with a radiolabeled riboprobe for detecting PSTVd plus strands. Lanes 1 and 4, mock-inoculated control; lanes 2, 3, 5, and 6, PSTVd-infected upper, noninoculated leaves collected at 25 days postinoculation. IPs were obtained with the antibody against AGO1 (lanes 4 and 6) or with a preimmune rabbit immunoglobulin fraction (IgG) (lane 5). Mock inoculations were performed with cultures of A. tumefaciens with a binary plasmid expressing GUS instead of the head-to-tail dimeric plus transcript of PSTVd. Accumulation of the PSTVd MC and ML forms was also examined in the RNA inputs after denaturing PAGE in 5% gels (upper gel). Equal loading was assessed by the intensity of the bands generated by the 5S RNA and tRNAs after staining with ethidium bromide.