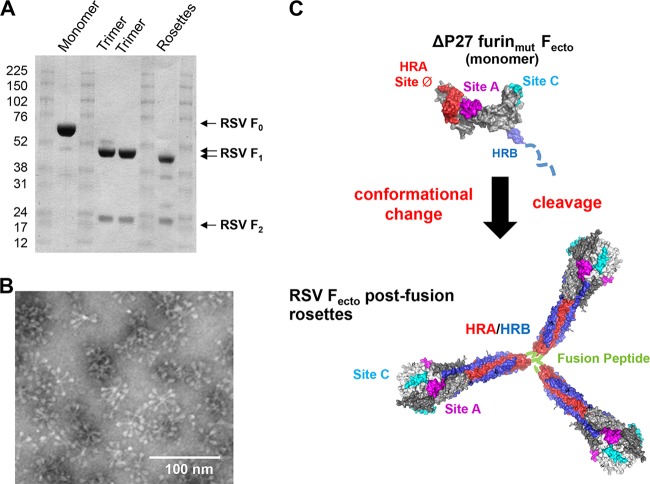
FIG 7.
Limited proteolysis of ΔP27 furinmut Fecto induces protein trimerization and association by the fusion peptide. (A) Sodium dodecyl sulfate-polyacrylamide gel showing limited proteolysis of RSV F constructs. RSV ΔP27 furinmut Fecto prior to digestion with trypsin is labeled “Monomer” and produces the F0 band. Two lots of RSV ΔFP furinwt Fecto are labeled “Trimer” and produce F1 and F2 bands in the absence of trypsin. RSV ΔP27 furinmut Fecto is labeled “Rosettes” and produces F1 and F2 bands after digestion with trypsin. (B) Electron microscopy image of the RSV Fecto rosettes formed spontaneously after trypsin treatment of ΔP27 furinmut Fecto. The rosettes have the elongated crutch shape of postfusion RSV Fecto, and proteins are associated at the end of the stalk where the hydrophobic fusion peptide is located. (C) Hypothetical model of ΔP27 furinmut Fecto as it undergoes conformational rearrangement after cleavage into the F1/F2 species. (Top) Model of uncleaved ΔP27 furinmut Fecto based on prefusion structure with Site A (sites II), Site C (site IV) and site Ø formed on the protein surface. HRB in blue is likely unfolded and is represented as a dashed line. A black arrow represents trypsin digestion of the monomer into F1/F2, leading to a conformational change of the prefusion-like monomer into the postfusion trimer. (Bottom) A model of RSV Fecto rosettes formed by postfusion trimers associated by interactions between their fusion peptides (green). Two neutralizing epitopes, Sites A and C, remain on the protein surface, while the prefusion site Ø epitope is lost as the HRA (red) is largely buried by the HRB (blue).