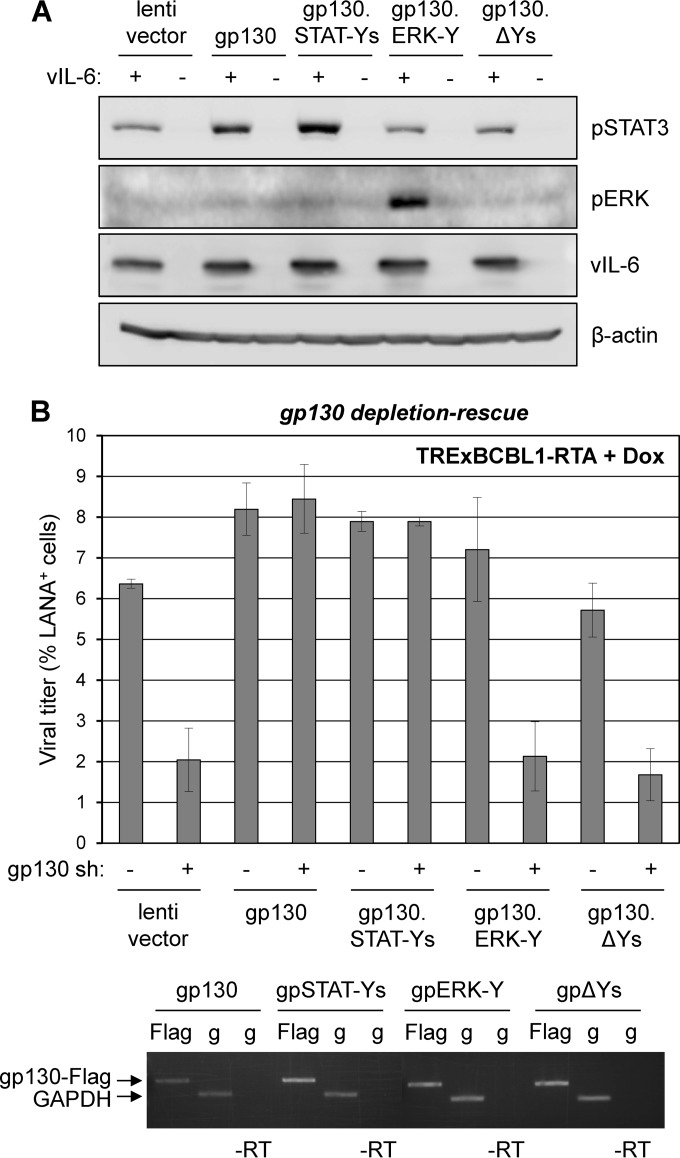
FIG 3.
Contributions of gp130-mediated STAT and ERK signaling to HHV-8 productive replication. (A) Functional assessment of gp130 signaling-tyrosine variants. HEK293T cells were cotransfected with empty vector (−) or vIL-6 plasmid (+) together with empty lentiviral vector or lentiviral vectors expressing wild-type gp130, gp130.STAT-Ys (Y759F-mutated), gp130.ERK-Y (Y759 only; other C-tail tyrosines mutated to F), or gp130.ΔYs (pan-Y-to-F-mutated). Two days postransduction, cells were lysed for Western blot analysis to detect phospho-STAT3 (pSTAT3) and pERK as indicators of gp130-activated STAT and MAPK pathways. (B) Role of STAT and ERK signaling in vIL-6/gp130 promotion of HHV-8 replication. TRExBCBL1-RTA PEL cells were transduced with empty lentiviral vector (pDUET001 [39]) or vectors expressing Flag-tagged wild-type gp130, gp130.STAT-Ys, gp130.ERK-Y, or gp130.ΔYs. Two days postransduction, cells were transduced with either NS (control) or gp130-specific shRNA-encoding lentiviral vectors. Following doxycycline-induced HHV-8 reactivation for 4 days, virus was collected and titers were determined as described previously. To verify appropriate expression of wild-type gp130 and the tyrosine-mutated gp130 variants, we lysed a subset of cells in TRIzol for preparation of RNA samples. RNA was reverse transcribed and amplified using 3′-Flag- and 5′-gp130-directed primers (TAGATGGCGGTGATGGTATTT and CTTGTCATCGTCGTCCTTGTA) to assess expression of transduced gp130-Flag mRNAs. GAPDH-specific primers were used for normalization and to provide a positive control. Flag, gp130-Flag RT-PCR; g, GADPH RT-PCR; −RT, no reverse transcriptase.