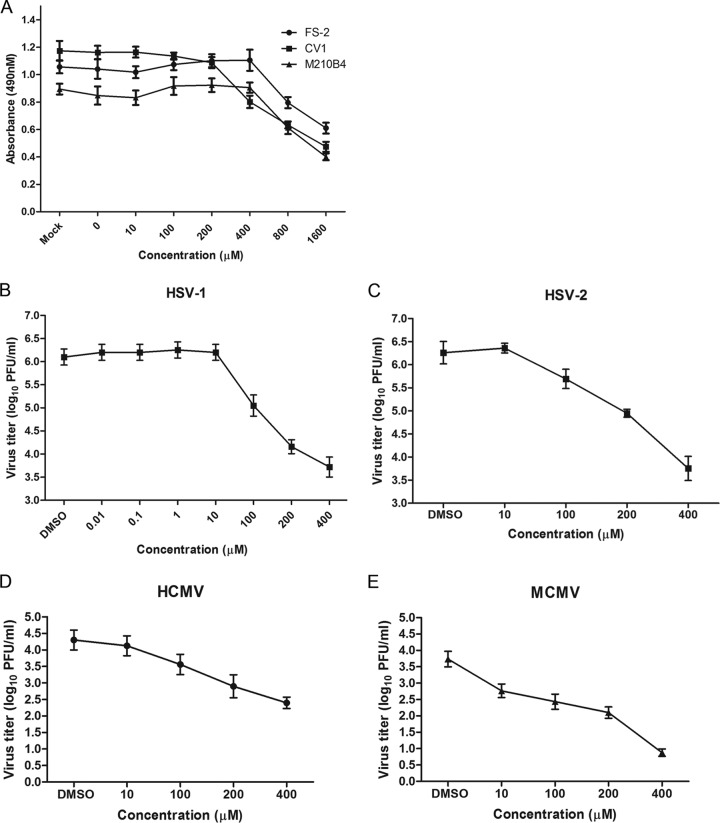
FIG 1.
Antiviral activities of raltegravir. (A) Cytotoxic effect of raltegravir. CV1, FS-2, and M210B4 cells at 80% confluence in 24-well plates were treated with various concentrations of raltegravir for 24 h. After treatment, a cell proliferation reagent (Promega) was added to each well, and 2 h later, the absorbance at 490 nm was recorded. (B and C) Specific anti-HSV-1 (B) and anti-HSV-2 (C) activity of raltegravir. Subconfluent CV1 cells in 6-well plates were infected with HSV-1(F) or HSV-2(G) at an MOI of 0.01 PFU per cell. After 60 min of incubation at 37°C, the inocula were removed, residual extracellular infectivity associated with the cells was reduced by treatment with a low-pH citrate buffer, and growth media containing the indicated concentrations of raltegravir were added. At 24 h after virus infection, the amount of infectious virus was determined by plaque assay in CV1 cells. (D) Specific anti-HCMV activity of raltegravir. Subconfluent FS-2 cells in 6-well plates were infected with HCMV at an MOI of 0.01 PFU per cell. After 60 min of incubation at 37°C, the inocula were removed, residual extracellular infectivity associated with the cells was reduced by treatment with a low-pH citrate buffer, and growth media containing the indicated concentrations of raltegravir were added. At 72 h after virus infection, the amount of infectious virus was determined by plaque assay in FS-2 cells. (E) Specific anti-MCMV activity of raltegravir. Subconfluent M210B4 cells in 6-well plates were infected with MCMV at an MOI of 0.01 PFU per cell. After 60 min of incubation at 37°C, the inocula were removed, residual extracellular infectivity associated with the cells was reduced by treatment with a low-pH citrate buffer, and growth media containing the indicated concentrations of raltegravir were added. At 72 h after virus infection, the amount of infectious virus was determined by plaque assay in M210B4 cells. In all panels, the data represent the means and standard errors of three replicates.