ABSTRACT
Vesicular stomatitis virus (VSV) has been extensively studied as a vaccine vector and oncolytic agent. Nevertheless, safety concerns have limited its widespread use in humans. The type III lambda interferon (IFN-λ) family of cytokines shares common signaling pathways with the IFN-α/β family and thus evokes similar antiviral activities. However, IFN-λ signals through a distinct receptor complex that is expressed in a cell type-specific manner, which restricts its activity to epithelial barriers, particularly those corresponding to the respiratory and gastrointestinal tracts. In this study, we determined how IFN-λ expression from recombinant VSV would influence vector replication, spread, and immunogenicity. We demonstrate that IFN-λ expression severely attenuates VSV in cell culture. In vivo, IFN-λ limits VSV replication in the mouse lung after intranasal administration and reduces virus spread to other organs. Despite this attenuation, however, the vector retains its capacity to induce protective CD8 T cell and antibody responses after a single immunization. These findings demonstrate a novel method of viral vector attenuation that could be used in both vaccine and oncolytic virus applications.
IMPORTANCE Viruses such as VSV that are used as vaccine vectors can induce protective T cell and antibody responses after a single dose. Additionally, IFN-λ is a potent antiviral agent that has certain advantages for clinical use compared to IFN-α/β, such as fewer patient side effects. Here, we demonstrate that IFN-λ attenuates VSV replication and spread following intranasal virus delivery but does not reduce the ability of VSV to induce potent protective immune responses. These findings demonstrate that the type III IFN family may have widespread applicability for improving the safety and efficacy of viral vaccine and oncolytic vectors.
INTRODUCTION
Generating protective immune responses after a single immunization can often be challenging using traditional, nonreplicating vaccine platforms. However, viral vaccine vectors can efficiently induce T cell and antibody responses that are necessary for effective protection from infection. Vesicular stomatitis virus (VSV), a negative-strand RNA virus, has been studied extensively as a vaccine vector and oncolytic agent (reviewed in reference 1), as it offers several advantages over other systems. For example, effective methods for recovering recombinant VSV vectors (2) and for expressing foreign genes from the VSV genome (3, 4) have been developed, and foreign antigen expression from the viral genome can lead to the development of protective T cell and antibody responses after a single dose. Human infection with VSV is rare and usually asymptomatic (5, 6), reducing the probability that preexisting immunity would limit the effectiveness of the vector. Additionally, VSV can potentially be delivered via a variety of immunization routes, including the intranasal route, which has the added advantage of needle-free administration.
Despite these advantages, the use of VSV as a vaccine vector in humans has been limited due to safety concerns, requiring further investigation into methods of attenuating the virus prior to its widespread deployment as a vaccine vector. Mutations or deletions of the viral glycoprotein severely attenuate viral pathogenesis and spread, but these mutations can also reduce the immunogenicity of the vector, especially when delivered intranasally (7–9). Vectors with mutations in the matrix protein that prevent viral evasion of the interferon (IFN) response maintain immunogenicity but still induce rapid weight loss in mouse models (10–14). Other methods of attenuating viral replication such as gene translocation (12, 13, 15, 16) and insertion of foreign genes into the VSV genome (7, 10, 13, 17–19) have also been employed with various degrees of success.
IFNs, including type I (IFN-α/β), type II (IFN-γ), and type III (IFN-λ1, -2, and -3; originally also known as interleukin-29 [IL-29], -28A, and -28B), are currently being investigated for their antiviral, immune-potentiating, and antitumor activities. IFN-λ, the most recently described IFN type, shares structural homology with the interleukin (IL)-10 family of cytokines (20–22) and signals through a unique receptor complex composed of the IFN-λR1 and IL-10R2 chains (20, 21). When activated, this receptor complex uses the same Jak-STAT signaling cascade as the type I IFN receptor and therefore exhibits many of the same antiviral and immune system-activating activities (23). In contrast to the ubiquitous expression of the IFN-α/β receptor, expression of the IFN-λ receptor chain is limited primarily to cells of the epithelial lineage (20, 21, 24–26), restricting IFN-λ antiviral activity to certain tissues, including the lung, intestine, and liver. In fact, IFN-λ plays a particularly important role in combating respiratory virus infections, as it is the dominant IFN produced in the lung (27–29). This signaling pattern of IFN-λ makes the cytokine a superior choice compared to type I IFNs in vaccination and therapeutic applications because it limits hematopoietic toxicities and other harmful side effects that are caused by systemic activation of IFN signaling cascades (30).
In this report, we demonstrate how expression of IFN-λ from the VSV genome affects vector replication, selectivity, and spread. IFN-λ expression significantly reduces viral replication in IFN-λ-responsive cell types. Additionally, vector replication is attenuated in the respiratory tract of mice when delivered intranasally, thus limiting systemic spread to other organs. However, despite reduction of VSV dissemination in vivo, the vector remains highly immunogenic compared to control nonattenuated vectors. Therefore, the results presented here demonstrate that expressing IFN-λ from VSV offers a unique approach to vector attenuation.
MATERIALS AND METHODS
Cell lines.
Hamster BHK-21 (BHK) epithelial cells and mouse BNL 1ME A.7R.1 (BNL) hepatocellular carcinoma cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 2 mM l-glutamine. Immortalized mouse hepatocytes (MMHD3) were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 100 ng/ml epidermal growth factor (BD Biosciences), 16 ng/ml insulin-like growth factor II, and 10 μg/ml insulin (Sigma, St. Louis, MO).
Plasmids and recombinant viruses.
The open reading frame of the murine IFN-λ2 gene (IL-28A) was codon optimized and chemically synthesized with the addition of the XhoI and NheI restriction sites at the 5′ and 3′ ends, respectively (GenScript). This IL-28 gene was subsequently cloned into the pVSV1XN plasmid or pVSVXN2 plasmid for expression from the 1st (VSV28.1) or 5th (VSV28.5) position of the VSV genome, respectively (4, 7).
Recombinant VSV expressing IFN-λ was recovered as previously described (2). Briefly, BHK cells were infected with vaccinia virus (VACV) expressing the T7 RNA polymerase and incubated for 1 h in serum-free DMEM. Infected cells were then transfected with the generated VSV genomes encoding IFN-λ2 and with expression vectors for the VSV N, P, and L proteins under the control of a T7 promoter. After 48 h, supernatants were filtered through a 0.2-μm-pore-size filter to remove VACV and passaged onto fresh BHK cells. Upon observation of cytopathic effects (∼2 days), the medium was collected and filtered through a 0.1-μm-pore-size filter. Recovered viruses were then plaque purified, expanded, and stored at −80°C until use. The titer of recovered virus stocks was determined by a plaque assay on BHK cells.
Empty recombinant VSV and VSV expressing green fluorescent protein (GFP) from the 1st position (VSVGFP.1) were provided by J. Rose (Yale University).
Reverse transcription (RT) and real-time PCR.
RNA was isolated from tissues using an RNeasy minikit (Qiagen, Valencia, CA), and 500 ng of RNA was reverse transcribed using a High Capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative PCR was performed using an Applied Biosystems 7500 real-time PCR system and SYBR green reaction mix (Applied Biosystems). Primer sequences were as follows: for VSV N, 5′-GAT AGT ACC GGA GGA TTG ACG ACT A-3′ (forward) and 5′-TCA AAC CAT CCG AGC CAT TC-3′ (reverse); and for GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-CAT GAG AAG TAT GAC AAC AGC CT (forward) and 5′-AGT CCT TCC ACG ATA CCA AAG T-3′ (reverse).
Infection procedures.
Female BALB/c mice 6 to 8 weeks of age were purchased from Charles River Laboratories (Wilmington, MA) and housed at the Yale University School of Medicine animal facilities. All experiments were performed in accordance with Yale Institutional Animal Care and Use Committee-approved procedures. Mice were lightly anesthetized with 30% isoflurane (Baxter) diluted in propylene glycol (vol/vol) and infected intranasally with 1 × 106 PFU of VSV, VSVGFP.1, VSV28.1, or VSV28.5 diluted in 25 μl of serum-free DMEM.
For experiments where viral RNA levels were determined, mice were infected as described above and euthanized at the indicated times via anesthetic overdose. Serum was collected, and tissues were excised and flash frozen in liquid nitrogen. Serum and tissues were stored at −80°C until further analysis.
Immunological assays.
For neutralization assays, serum was serially diluted in phosphate-buffered saline (PBS) in a 96-well plate. Dilutions were then incubated with 100 PFU of VSV for 1 h at 37°C to allow serum antibodies to bind virus. The neutralization mixture was then added to 96-well tissue culture plates with 4 × 103 BHK cells in DMEM. Plates were subsequently incubated at 37°C for 72 h and observed for cytopathic effects. Antibody titers were determined according to the lowest serum dilution that yielded 100% neutralization of VSV.
Memory T cell responses were determined using an IFN-γ enzyme-linked immunospot (ELISPOT) set (BD Biosciences) following the manufacturer's protocol. Briefly, 96-well plates were coated overnight with purified anti-mouse IFN-γ antibody (1:200). Plates were rinsed and then blocked for 2 h using DMEM supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 2 mM l-glutamine. Splenocytes were purified from mice by passaging spleens through 70-μm-pore-size strainers (BD Falcon) and treating the cell suspension with ACK lysing buffer (Lonza). After being washed with Hanks' balanced salt solution (Invitrogen), cells were suspended in DMEM and seeded at 2 × 105 cells/well. A VSV N peptide (MPYLIDFGL [31]) was used at 10 μg/ml to stimulate the cells overnight at 37°C. Cells were washed from plates using PBS-Tween (0.05% [vol/vol]), and biotinylated anti-mouse IFN-γ antibody (1:250) was added for 2 h at 25°C. After washing, streptavidin-horseradish peroxidase (HRP) (1:100) was added to wells and incubated for 1 h at 25°C. Following the final washes, 3-amino-9-ethyl-carbazole (AEC) chromogen substrate (BD Biosciences) was added to the wells and allowed to develop at 25°C for 20 to 40 min. Distilled water was added to stop the reaction, and the plates were allowed to air dry before spot-forming cells (SFC) were counted.
IFN-λ detection and activity.
BHK or MMHD3 cells were infected with VSV, VSV28.1, or VSV28.5 (multiplicity of infection [MOI] = 1), and samples were collected at the indicated time points. Media were collected and either mixed with 2× SDS sample buffer or used to treat MMHD3 cells to determine STAT1 activation results. Attached cells were washed once with PBS and collected in 500 μl of 2× SDS sample buffer. Proteins were separated on a 10% SDS gel, transferred to a nitrocellulose membrane, and probed with anti-mouse IFN-λ (Santa Cruz Biotechnologies) or anti-phospho-STAT1 (Tyr701; Cell Signaling Technology). Blots were subsequently stripped and reprobed with anti-actin (Santa Cruz Biotechnologies), anti-VSV (a gift from the laboratory of J. Rose), or anti-STAT1 (Cell Signaling Technology) antibodies. Appropriate secondary antibodies were used for detection, and blots were developed using chemiluminescence.
Statistical data analysis.
Student's t test was used to determine significant differences in virus plaque sizes, virus titers, and CD8 T cell responses. P values of <0.05 were considered statistically significant.
RESULTS
Generation of VSV vectors expressing functionally active IFN-λ.
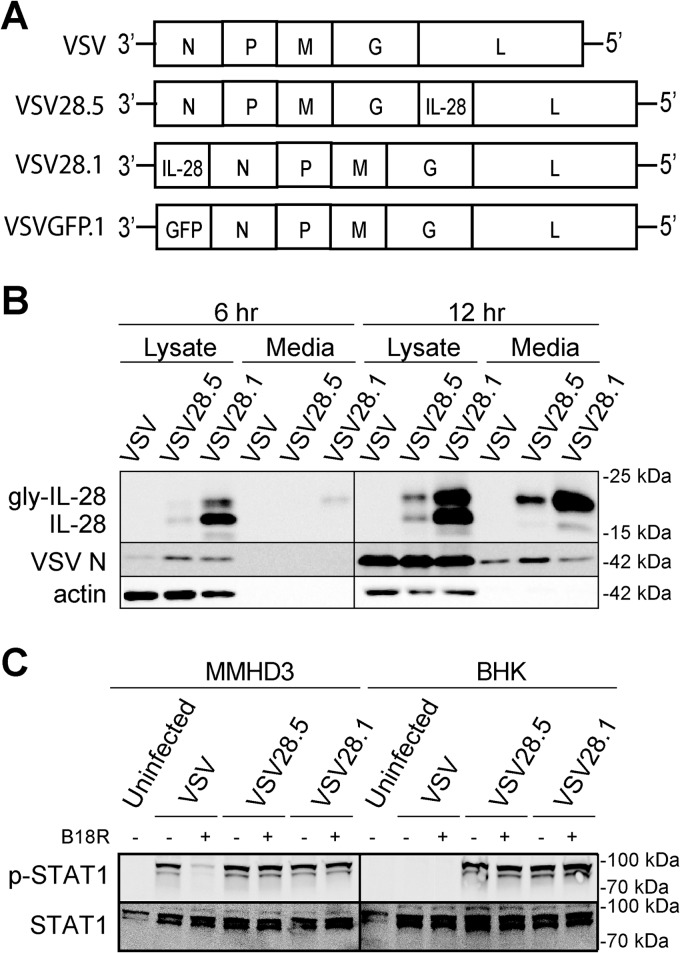
The VSV genome sequentially encodes 5 proteins (3′-N, P, M, G, L-5′), which are transcribed in the indicated order in decreasing abundance (32–34). We initially chose to make a VSV that expresses IFN-λ from the 5th position (VSV28.5; Fig. 1A), as these viruses are typically easier to recover (J. Rose, personal communication). However, since the level of VSV mRNA synthesis decreases sequentially for each viral protein (35), successful expression from the 1st position of the genome can lead to levels of protein synthesis that are more than 3-fold higher (7). Therefore, to maximize secretion of IFN-λ from our VSV vector, we also generated a recombinant VSV vector that expresses IFN-λ from the 1st position (VSV28.1; Fig. 1A). Control viruses included empty recombinant VSV (VSV) and VSV encoding GFP from the 1st position (VSVGFP.1; Fig. 1A). We confirmed that IFN-λ is indeed expressed and secreted at greater levels from VSV28.1-infected cells than from the VSV28.5-infected cells at both 6 and 12 h postinfection (p.i.) of BHK cells (Fig. 1B).
FIG 1.
Expression of functionally active IFN-λ (IL-28) protein from VSV. (A) Genomic diagrams of the VSV vectors generated in this study. All five genes encoding the nucleocapsid (N), the phosphoprotein (P), the matrix protein (M), the glycoprotein (G), and the RNA-dependent RNA polymerase (L) were present in the viruses. The mouse IFN-λ2 (IL-28A) gene was inserted either upstream of the L gene in the 5th position (VSV28.5) or upstream of the N gene in the 1st position (VSV28.1). Control viruses with either no insert (VSV) or the GFP gene inserted in the 1st position (VSVGFP.1) are also depicted. (B) Immunoblot analysis of glycosolated or unglycosolated IFN-λ protein expressed in the lysates or media collected from BHK cells infected with the designated viruses at either 6 or 12 h p.i. Molecular mass values are indicated. (C) Immunoblot of STAT1 (p-STAT1α [91 kDa] and a splice variant, STAT1β [84 kDa]) phosphorylation in MMHD3 cells incubated for 30 min with media from either MMHD3 or BHK cells collected after a 24-h infection with VSV, VSV28.5, or VSV28.1. Media were treated with or without soluble B18R protein to block IFN-α/β before transfer onto MMHD3 cells as designated. Molecular mass values are indicated.
We next determined if the vector-expressed IFN-λ is functionally active by measuring its ability to stimulate STAT1 phosphorylation. Media from VSV-, VSV28.5-, and VSV28.1-infected MMHD3 cells all stimulated STAT1 phosphorylation when transferred onto IFN-λ-responsive MMHD3 cells (Fig. 1C). To show that the phosphorylation of STAT1 is due specifically to a type III IFN response initiated by IFN-λ in VSV28.5- and VSV28.1-infected cells, we measured STAT1 phosphorylation in the presence and absence of VACV B18R protein. The secreted B18R protein shares significant regions of homology with the IFN-α/β receptor and therefore effectively blocks type I, but not type III, IFN signaling (36). Recombinant B18R was added to the media collected from MMHD3 cells infected with the VSV vectors before the media were transferred to fresh MMHD3 cells. The results clearly demonstrate that STAT1 phosphorylation in the presence of B18R was substantially reduced only when MMHD3 cells were treated with media from VSV-infected cells and not when they were treated with media from VSV28.5- or VSV28.1-infected cells, indicating that phosphorylation of STAT1 resulted from a type III IFN response induced by vector-expressed IFN-λ (Fig. 1C).
This experiment was also performed using BHK cells for the initial infection by either VSV or the VSV28 vectors. BHK cells are defective in their IFN signaling pathways and consequently do not produce IFNs after infection with the VSV vectors. In contrast to MMHD3 cells, STAT1 phosphorylation was observed only in VSV28.5- and VSV28.1-infected cells and was not observed in VSV-infected cells regardless of the presence or absence of B18R (Fig. 1C). Thus, we conclude that IFN-λ is expressed in greater amounts from the 1st-position vector, is functionally active, and specifically stimulates type III IFN signaling.
VSV28 vectors are attenuated in IFN-λ-responsive cells.
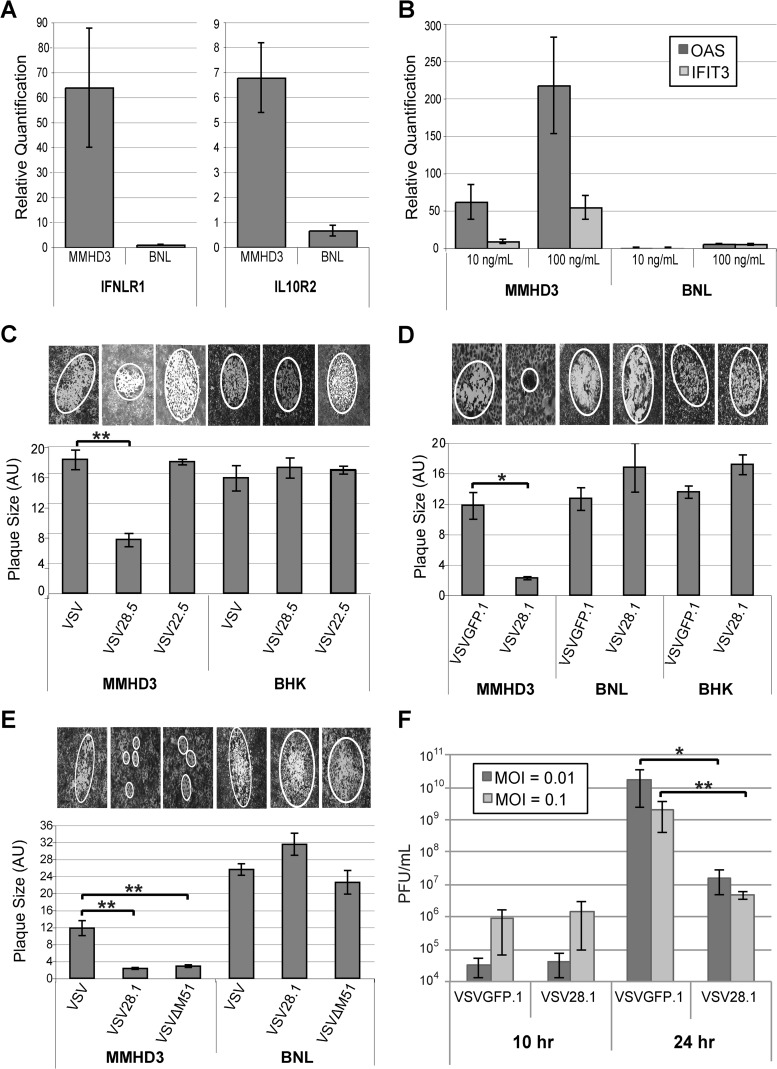
To determine if IFN-λ expression from the VSV28.5 and VSV28.1 vectors impacts their replication, cell lines were chosen that do (IFN-λ responsive) or do not (IFN-λ nonresponsive) express IFN-λR1. Mouse BNL hepatoma cells do not express IFN-λR1 and therefore do not directly respond to IFN-λ (37). To model normal cells in cell culture, the mouse immortalized hepatocyte MMHD3 cell line was chosen (38). These cells require exogenous growth factors for survival, can be differentiated in culture, and display many properties of normal hepatocytes, including responsiveness to the type III IFNs (39). As expected, consistent with the differential sensitivities of these cell lines to IFN-λ, there were approximately 60-fold-higher mRNA levels of IFN-λR1 and 6-fold-higher levels of IL-10Rβ mRNA in MMHD3 cells than in BNL cells (Fig. 2A).
FIG 2.
VSV expressing IFN-λ is attenuated in IFN-λ-responsive cells. (A) Total RNA was prepared from MMHD3 or BNL cells, and IL-10Rβ and IFN-λR1 mRNA was quantified by quantitative RT-PCR. (B) MMHD3 or BNL cells were treated with or without 10 ng/ml or 100 ng/ml IFN-λ for 24 h and harvested for total RNA isolation. ISGs OAS and IFIT3 were quantified by quantitative RT-PCR. (C to E) MMHD3, BNL, or BHK cells were infected with serially diluted virus, and at 2 days p.i., plaques were stained and then visualized and photographed under a light microscope for quantification of the average relative plaque size by measuring the area of an ellipse that was fitted to the plaque. The bar graphs depict the mean individual plaque areas, with error bars representing the standard errors of the means. Representative images of plaques are shown above each graph. (F) MMHD3 cells were infected with VSVGFP.1 or VSV28.1 at MOIs of 0.01 and 0.1, and media were collected at 10 and 24 h p.i. to quantify viral production by a standard plaque assay. *, P < 0.05; **, P < 0.01.
In order to further investigate the IFN-λ sensitivity of these cell lines, we tested the ability of IFN-λ to induce expression of IFN-stimulated genes (ISGs). We stimulated BNL and MMHD3 cells with 10 or 100 ng/ml of IFN-λ for 24 h and determined the relative mRNA amounts of representative ISGs using quantitative RT-PCR. The canonical ISGs OAS and IFIT3 were efficiently induced by 10 or 100 ng/ml of IFN-λ in MMHD3 cells but not in BNL cells (Fig. 2B), consistent with the different levels of receptor expression in these cell types.
We found that the VSV28.5 virus is attenuated in IFN-λ-responsive MMHD3 cells compared to VSV but not in cells that are IFN-λ nonresponsive (hamster BHK cells), as measured by relative virus plaque sizes in culture following low-MOI infection (Fig. 2C; images of typical plaques in MMHD3 or BHK cells are shown). VSV expressing the structurally related IL-22 cytokine from the 5th position (VSV22.5) exhibited plaque sizes comparable to those of VSV. Thus, the attenuation (reduced plaque size) of VSV28.5 that we observed in the MMHD3 cells is the result of IFN-λ expression and not the result of inserting the cytokine into the 5th position of the viral genome.
In similarly conducted plaque size assays, we found that VSV28.1 was attenuated compared to a control VSV vector expressing GFP from the 1st position (VSVGFP.1) as measured by relative plaque sizes in IFN-λ-responsive MMHD3 cells but not in IFN-λ-nonresponsive mouse BNL or hamster BHK cells (Fig. 2D; images of typical plaques in MMHD3 cells, BHK cells, and BNL cells are shown). In the nonresponsive BNL and BHK cells, there was no reduction in VSV28.1 plaque sizes compared to VSVGFP.1 plaque sizes, indicating that placement of the IFN-λ coding sequence in the first genome position is not inherently detrimental to VSV replication. However, in the IFN-λ-responsive MMHD3 cells, we found a significant reduction in plaque size with the VSV28.1 virus to the extent that it was difficult to visual the plaques, which could more accurately be described as foci of cells displaying virus-induced cytopathic effects rather than as true plaques (Fig. 2D).
A number of studies have centered on ways to further improve the safety and selectivity of VSV for clinical use. The VSV matrix (M) protein, in addition to being a viral structural protein, also binds to the nuclear pore complex and prevents export of cellular mRNA. Specific point mutations in the M protein (such as deletion of methionine at position 51 [ΔM51]) abrogate the ability of M protein to block mRNA export and, as a consequence, make VSV hypersensitive to the cellular IFN response. Stojdl and colleagues first demonstrated that VSVΔM51 not only retains the ability to kill cancer cells but also is attenuated in normal cells and mice (40), a finding that was subsequently confirmed in other tumor models (41–43). We therefore wanted to compare the attenuation levels of our VSV28.1 vector and the well-characterized VSVΔM51 vector. We found that the VSV28.1 virus displayed a reduction in plaque size in IFN-sensitive MMHD3 cells similar to that seen with VSV encoding the ΔM51 mutation (Fig. 2E). Therefore, VSV28.1 shows a level of attenuation in IFN-responsive cells similar to that seen with the highly attenuated ΔM51 mutant.
Since VSV28.1 was significantly more attenuated in IFN-responsive cells than VSV28.5, we also examined VSV28.1 in replication assays measuring virus production over time. In these assays, the amount of VSV28.1 virus produced by 10 or 24 h p.i. at MOIs of 0.01 and 0.1 was measured in the IFN-λ-responsive MMHD3 cells. We found a >2-log decrease in replication for the VSV28.1 virus compared to the control VSVGFP.1 virus by 24 h p.i. (Fig. 2F). In conclusion, we established that the VSV28 vectors are clearly attenuated in IFN-λ-responsive cells.
VSV28.1 is attenuated in vivo and yet induces protective immune responses.
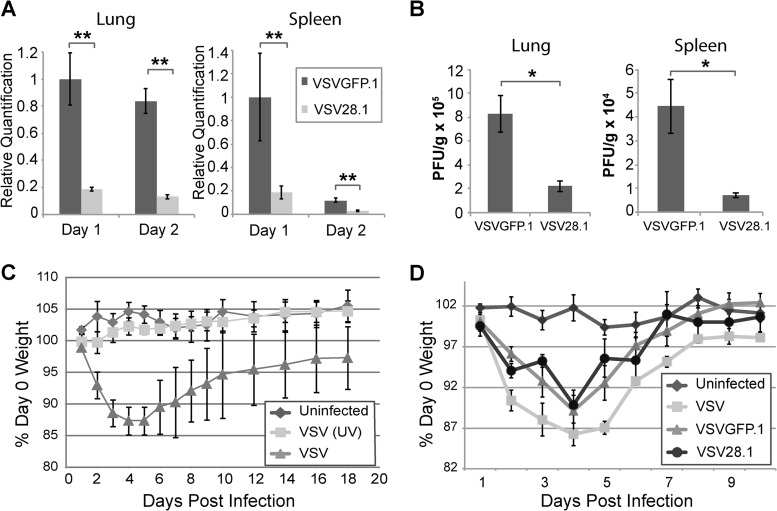
As we found that VSV28.1 was significantly more attenuated in cell culture than VSV28.5, we focused our in vivo analyses on VSV28.1. After intranasal infection with recombinant VSV, the virus initially replicates in the lung and subsequently spreads to peripheral organs via the blood (44). However, unlike naturally derived wild-type (WT) VSV isolates, which can also spread throughout the brain (45), recombinant VSV is efficiently controlled in the olfactory bulb by the host type I IFN response (46, 47). To directly measure virus replication in the lung and peripheral organs, mice were infected by the intranasal route with 1 × 106 PFU of either VSVGFP.1 or VSV28.1, and viral RNA (Fig. 3A) or titers (Fig. 3B) were measured by quantitative RT-PCR at both day 1 and day 2 or by plaque assay at day 1 p.i. in the lung and spleen. VSV28.1 levels were markedly reduced in both the lung and spleen relative to VSVGFP.1 levels. This trend of comparatively reduced RNA levels was also observed in the liver and cervical lymph node, although the difference between the levels in the two viruses did not reach statistical significance in these tissues (data not shown). As previously reported (48), we observed no pathology in the lung following intranasal VSV infection (data not shown). Thus, we conclude that the 1st position VSV28.1 vector is attenuated for replication and spread in vivo.
FIG 3.
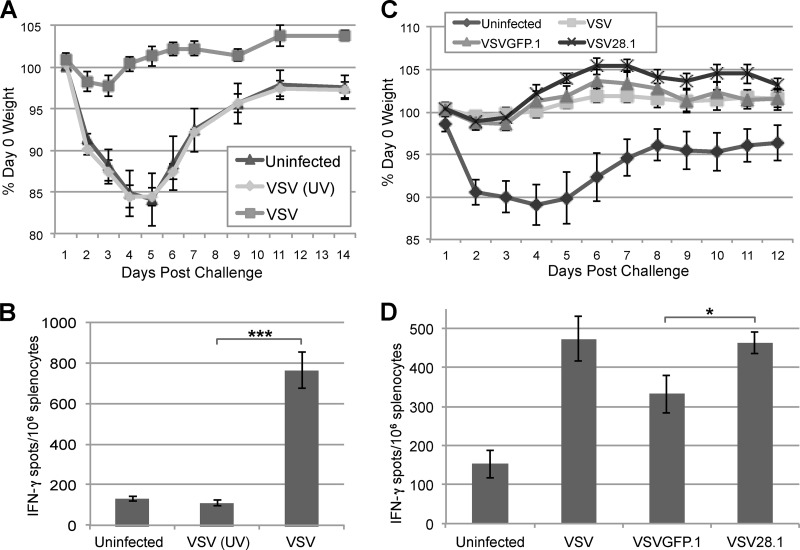
Reduced replication and spread of VSV28.1 following intranasal infection of mice. (A and B) Mice were intranasally infected with 1 × 106 PFU of VSVGFP.1 or VSV28.1. The lungs and spleen were harvested, and viral genomic RNA (VSV-N) (A) or viral titers (B) were quantified by quantitative RT-PCR or by a plaque assay, respectively. (C and D) Mice were left uninfected or intranasally infected with 1 × 106 PFU of VSV or its UV-inactivated equivalent (C) or 1 × 106 PFU of VSV, VSVGFP.1, or VSV28.1 (D). Average weight loss was measured daily until mice had recovered. Error bars represent the standard errors of the means (n = 5). *, P < 0.05; **, P < 0.01.
Although the systemic infection that follows intranasal infection with recombinant WT VSV is typically not fatal, mice are known to lose 10% to 15% of their body weight by day 3 to day 5 p.i. due to induction of tumor necrosis factor alpha (TNF-α) (14). The mice subsequently regain weight, reaching preinfection levels by day 10 to 20. This assay has previously been employed to measure attenuation of other VSV vectors (7, 8). We first tested whether viral replication is necessary for induction of weight loss in this model. BALB/c mice were intranasally immunized with 1 × 106 PFU of VSV or its equivalent of UV-inactivated VSV, and mice were weighed for 18 days p.i. No measurable weight changes were observed in mice immunized with UV-inactivated virus (Fig. 3C), suggesting that VSV replication is required to induce TNF-α and subsequent weight loss during VSV infection. To test whether weight loss induced by VSV28.1 differed from that induced by VSVGFP.1, mice intranasally infected with 1 × 106 PFU of VSV, VSVGFP.1, or VSV28.1 were weighed over 10 days. Similar levels of initial VSV-induced weight loss were observed with the VSV, VSVGFP.1, and VSV28.1 vectors (Fig. 3D), indicating comparable levels of early TNF-α production after infection with these viruses.
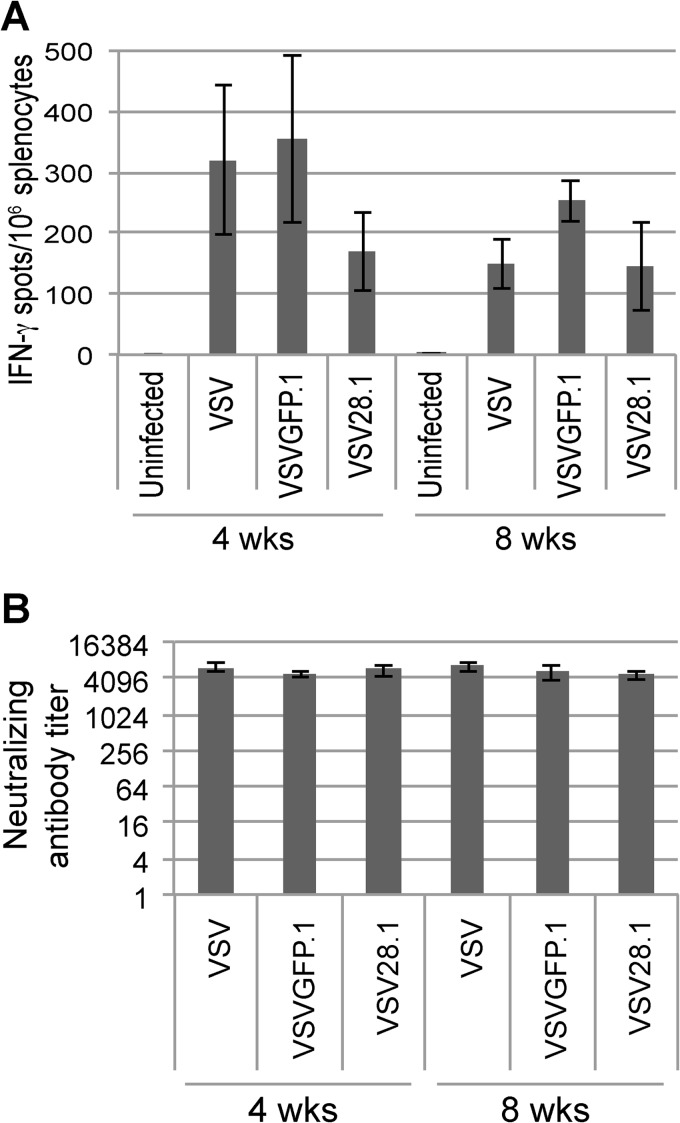
At 4 and 8 weeks p.i., we examined the immune response to VSV generated in the mice in two ways. First, we assessed the memory CD8 T cell response to the immunodominant epitope of the VSV N protein (Fig. 4A); second, we assessed the neutralizing antibody response induced to the VSV G protein (Fig. 4B). We found that despite the reduced replication and spread exhibited by VSV28.1 in the lung and spleen (Fig. 3A and B), compared to the VSV and VSVGFP.1 vectors, the VSV28.1 virus generated robust although moderately lower CD8 T cell (Fig. 4A) and comparable neutralizing antibody (Fig. 4B) responses in infected animals. Thus, despite its marked attenuation, the VSV28.1 vector retains its ability to induce potent immune responses.
FIG 4.
VSV28.1 induces CD8 T cell and antibody responses similar to those of nonattenuated vectors. Mice were intranasally infected with 1 × 106 PFU of VSV, VSVGFP.1, or VSV28.1. At 4 and 8 weeks p.i., the splenic VSV-specific CD8 T cells were quantified by IFN-γ ELISPOT assays (A), and VSV neutralizing antibody titers were measured in the serum (B). Error bars represent standard errors of the means (n = 5).
Because we observed somewhat reduced CD8 T cell responses to VSV28.1, we next analyzed the protective potential of the memory T cells elicited by this virus. Furthermore, since we observed reduced viral titers in mice after VSV28.1 infection, we also determined if replication is required to induce protection from challenge. Mice were left uninfected or were intranasally immunized with either 1 × 106 PFU of VSV or its equivalent of UV-inactivated VSV (Fig. 5A and B) or with 1 × 106 PFU of VSV, VSVGFP.1, or VSV28.1 (Fig. 5C and D). One month later, all mice were challenged intranasally with 1 × 106 PFU of a VSV encoding the Chandipura serotype G protein VSV(G)ch, which is not neutralized by antibody generated to the parental Indiana serotype VSV strain. Postchallenge, the mice were weighed every 1 to 2 days, and average weights from each group are shown in Fig. 5A and C. Only the groups that were originally uninfected or immunized with UV-inactivated virus exhibited weight loss after VSV(G)ch challenge (Fig. 5A and C). In contrast, mice that were immunized with VSV, VSVGFP.1, or VSV28.1 were all protected from weight loss following challenge (Fig. 5A and C).
FIG 5.
VSV28.1 immunization protects from subsequent virus challenge. (A and C) Mice were immunized intranasally with PBS (Uninfected), 1 × 106 PFU of VSV, or the equivalent of 1 × 106 PFU of UV-inactivated VSV (A) or with PBS, VSV, VSVGFP.1, or VSV28.1 (C). At 1 month p.i., all groups were challenged with 1 × 106 PFU of VSV(G)ch, and weight loss was measured for 2 weeks postchallenge. The average weight for each group is shown. (B and D) Splenocytes collected from mice 2 weeks postchallenge were assayed to quantify splenic VSV-specific CD8 T cells by IFN-γ ELISPOT assays (n = 5). *, P < 0.05; ***, P < 0.001.
Splenocytes from these mice were then subjected to IFN-γ ELISPOT assays 2 weeks postchallenge to measure CD8 T cell recall responses (Fig. 5B and D). The CD8 T cell responses in mice initially infected with VSV, VSVGFP.1, and VSV28.1 were all higher than the CD8 T cell responses exhibited by unimmunized mice or mice immunized with UV-inactivated virus, consistent with the observed protection of the animals from weight loss. These results indicate that although replication is required for induction of T cell responses with protective properties, the reduction in viral titers and primary T cell responses exhibited by VSV28.1 does not affect its ability to protect mice from challenge.
DISCUSSION
Viral vaccine vectors induce strong and often protective antibody and T cell responses, and yet their use in humans has been limited mainly due to safety concerns. In this study, we demonstrated that IFN-λ expression during administration of a VSV-based vaccine vector helps to minimize spread while maintaining the immunogenicity of the vector. Many vaccines focus on generating protective antibodies since antibodies act early to bind, neutralize, and clear entering pathogens. In some cases, however, T cell responses could play a significant role in protection (recently reviewed in reference 49). Although we observed a slight decrease in T cell responses to VSV28.1 compared to VSVGFP.1 at 4 and 8 weeks p.i., these responses were still far above what we observe in uninfected mice and were protective against subsequent virus challenge. Additionally, neutralizing antibody responses remained equivalent to those seen with the nonattenuated viruses. Therefore, we expect that IFN-λ expression would not limit VSV's efficacy as a vaccine vector. However, although we did not investigate whether different or weaker T cell epitopes would be more significantly affected by vector attenuation mediated by IFN-λ expression, this possibility is a topic for future investigation.
Despite the fact that VSV28.1 displayed reduced replication and subsequent spread compared to control VSVGFP.1 in infected mice, the animals lost similar amounts of weight after infection. Weight loss following intranasal VSV infection is abrogated in the absence of TNF-α (14), implicating the cytokine in this process. However, the similar weight loss phenotypes observed here were not entirely unexpected for two reasons. First, the initial round of virus infection and replication of VSV28.1 is likely to be similar to that of VSVGFP.1, as IFN-λ protein expression and accumulation would be necessary to reduce virus replication and spread. In fact, this can be observed in our in vitro data, where little reduction in virus is seen early after infection (Fig. 2F). Second, an important cellular source of TNF-α may be virus-specific T cells, which we find are similar in VSVGFP.1- and VSV28.1-infected animals.
Although attenuating VSV vectors can result in reduced immunogenicity, the immunostimulatory properties of IFN-λ may help VSV28.1 maintain its ability to induce protective immune responses. In DNA vaccine studies, IFN-λ expression augments CD8 T cell responses while preventing development of regulatory T cells (50–52). However, the mechanism by which IFN-λ acts to promote immune responses remains unclear. Certain subsets of immune cells express the IFN-λ receptor (53, 54), but IFN-λ signaling in epithelial cells may also contribute indirectly to immune activation. Furthermore, since IFN-λ can in some cases induce a tolerogenic phenotype in immune cells (54), the potential adjuvant properties of the cytokine may depend on the inflammatory environment present during expression.
Due to its neurotropic properties, the degree of attenuation required for VSV's use in humans might rise above that which we achieved through expression of IFN-λ. For example, although we did not observe any overt neuropathogenicity in our experiments, we were still able to detect some viral genomes in the brains of mice (data not shown). Additional methods of VSV attenuation could easily be employed in combination with IFN-λ expression. For instance, matrix protein mutations, which interfere with VSV's ability to shut down host antiviral defenses (40), may further attenuate the vector while still allowing robust expression of IFN-λ. Furthermore, encoding IFN-λ may be a useful strategy for attenuating other viral vaccines or vaccine vectors. For example, some adenovirus vaccines consist of an orally delivered, enteric live virus that effectively protects against the acute respiratory distress syndrome associated with certain adenovirus strains. Despite the success of these vaccines, occasional viral transmission to unvaccinated contacts (55–57) and replication in the respiratory tract of vaccinated individuals with some adenovirus strains (57, 58) suggests that the development of attenuated vaccine vectors for this infection would be beneficial. Additionally, many replication-incompetent adenovirus-based vaccine- and gene-delivery vectors can undergo homologous recombination during production to generate replication-competent virus contaminants (59, 60). Using IFN-λ to attenuate adenovirus vaccines and vectors may help reduce viral replication in the respiratory tract and/or intestinal epithelium and thus improve the safety profiles of these vaccines and gene-delivery vehicles.
In addition to the antiviral activities of IFN-λ, the cytokine also exhibits antitumor properties. In mouse models of melanoma, IFN-λ promotes cell cycle arrest and apoptosis in vitro and reduces tumor vascularity, slows growth, and prevents metastasis in vivo (37, 61). Similarly, in models of hepatoma and fibrosarcoma, IFN-λ prevents tumor growth and metastasis (62, 63). The in vivo antitumor activity of IFN-λ in these studies was mediated mainly by the indirect recruitment and activation of natural killer and CD8 T cells rather than by its antiproliferative effects (61–63). Overall, the antitumor properties of IFN-λ combined with VSV's propensity to specifically target tumors suggest that the VSV28.1 vector presented in this study may also be useful in cancer immunotherapies.
ACKNOWLEDGMENTS
Research reported in this publication was supported by a Pilot Project grant from the Yale Cancer Center and the NCI of the NIH under award number R21CA175802. R.C.G. and T.D.R. were also supported by NIH NIAID training grant T32AI055403, and X.W. was supported by a fellowship from the China Scholarship Council.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published ahead of print 9 July 2014
REFERENCES
- 1.Finke S, Conzelmann KK. 2005. Recombinant rhabdoviruses: vectors for vaccine development and gene therapy. Curr. Top. Microbiol. Immunol. 292:165–200. 10.1007/3-540-27485-5_8 [DOI] [PubMed] [Google Scholar]
- 2.Lawson ND, Stillman EA, Whitt MA, Rose JK. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 92:4477–4481. 10.1073/pnas.92.10.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnell MJ, Buonocore L, Kretzschmar E, Johnson E, Rose JK. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. U. S. A. 93:11359–11365. 10.1073/pnas.93.21.11359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnell MJ, Buonocore L, Whitt MA, Rose JK. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesh RB, Peralta PH, Johnson KM. 1969. Ecologic studies of vesicular stomatitis virus. I. Prevalence of infection among animals and humans living in an area of endemic VSV activity. Am. J. Epidemiol. 90:255–261 [DOI] [PubMed] [Google Scholar]
- 6.Johnson KM, Vogel JE, Peralta PH. 1966. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV). Am. J. Trop. Med. Hyg. 15:244–246 [DOI] [PubMed] [Google Scholar]
- 7.Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, Rose JK. 2005. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J. Virol. 79:15043–15053. 10.1128/JVI.79.24.15043-15053.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Publicover J, Ramsburg E, Rose JK. 2004. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J. Virol. 78:9317–9324. 10.1128/JVI.78.17.9317-9324.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollmann G, Rogulin V, Simon I, Rose JK, van den Pol AN. 2010. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J. Virol. 84:1563–1573. 10.1128/JVI.02040-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M, Cramer SD, Lyles DS. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34–49. 10.1016/j.virol.2004.08.039 [DOI] [PubMed] [Google Scholar]
- 12.Clarke DK, Nasar F, Lee M, Johnson JE, Wright K, Calderon P, Guo M, Natuk R, Cooper D, Hendry RM, Udem SA. 2007. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 81:2056–2064. 10.1128/JVI.01911-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper D, Wright KJ, Calderon PC, Guo M, Nasar F, Johnson JE, Coleman JW, Lee M, Kotash C, Yurgelonis I, Natuk RJ, Hendry RM, Udem SA, Clarke DK. 2008. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and g gene truncation reduces neurovirulence and enhances immunogenicity in mice. J. Virol. 82:207–219. 10.1128/JVI.01515-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Publicover J, Ramsburg E, Robek M, Rose JK. 2006. Rapid pathogenesis induced by a vesicular stomatitis virus matrix protein mutant: viral pathogenesis is linked to induction of tumor necrosis factor alpha. J. Virol. 80:7028–7036. 10.1128/JVI.00478-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanagan EB, Schoeb TR, Wertz GW. 2003. Vesicular stomatitis viruses with rearranged genomes have altered invasiveness and neuropathogenesis in mice. J. Virol. 77:5740–5748. 10.1128/JVI.77.10.5740-5748.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan EB, Zamparo JM, Ball LA, Rodriguez LL, Wertz GW. 2001. Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J. Virol. 75:6107–6114. 10.1128/JVI.75.13.6107-6114.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wertz GW, Moudy R, Ball LA. 2002. Adding genes to the RNA genome of vesicular stomatitis virus: positional effects on stability of expression. J. Virol. 76:7642–7650. 10.1128/JVI.76.15.7642-7650.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J, Bidula SM, Jensen TM, Reiss CS. 2009. Cytokine-modified VSV is attenuated for neural pathology, but is both highly immunogenic and oncolytic. Int. J. Infereron Cytokine Mediator Res. 1:15–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Pol AN, Davis JN. 2013. Highly attenuated recombinant vesicular stomatitis virus VSV-12′GFP displays immunogenic and oncolytic activity. J. Virol. 87:1019–1034. 10.1128/JVI.01106-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69–77. 10.1038/ni875 [DOI] [PubMed] [Google Scholar]
- 21.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63–68. 10.1038/ni873 [DOI] [PubMed] [Google Scholar]
- 22.Gad HH, Dellgren C, Hamming OJ, Vends S, Paludan SR, Hartmann R. 2009. Interferon-lambda is functionally an interferon but structurally related to the interleukin-10 family. J. Biol. Chem. 284:20869–20875. 10.1074/jbc.M109.002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagliaccetti NE, Robek MD. 2010. Interferon-lambda in HCV infection and therapy. Viruses 2:1589–1602. 10.3390/v2081589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommereyns C, Paul S, Staeheli P, Michiels T. 2008. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4:e1000017. 10.1371/journal.ppat.1000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K. 2009. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 10:702–714. 10.1038/gene.2009.72 [DOI] [PubMed] [Google Scholar]
- 26.Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, Staeheli P. 2008. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 4:e1000151. 10.1371/journal.ppat.1000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jewell NA, Cline T, Mertz SE, Smirnov SV, Flaño E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. 2010. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J. Virol. 84:11515–11522. 10.1128/JVI.01703-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Günther S, Drosten C, Michiels T, Staeheli P. 2010. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 84:5670–5677. 10.1128/JVI.00272-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okabayashi T, Kojima T, Masaki T, Yokota S, Imaizumi T, Tsutsumi H, Himi T, Fujii N, Sawada N. 2011. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 160:360–366. 10.1016/j.virusres.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 30.Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Lopez-Talavera JC, Byrnes-Blake K, Fontana D, Freeman J, Gray T, Hausman D, Hunder NN, Lawitz E. 2010. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology 52:822–832. 10.1002/hep.23743 [DOI] [PubMed] [Google Scholar]
- 31.Ramsburg EA, Publicover JM, Coppock D, Rose JK. 2007. Requirement for CD4 T cell help in maintenance of memory CD8 T cell responses is epitope dependent. J. Immunol. 178:6350–6358. 10.4049/jimmunol.178.10.6350 [DOI] [PubMed] [Google Scholar]
- 32.Abraham G, Banerjee AK. 1976. Sequential transcription of the genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. U. S. A. 73:1504–1508. 10.1073/pnas.73.5.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ball LA, White CN. 1976. Order of transcription of genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. U. S. A. 73:442–446. 10.1073/pnas.73.2.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villarreal LP, Breindl M, Holland JJ. 1976. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry 15:1663–1667. 10.1021/bi00653a012 [DOI] [PubMed] [Google Scholar]
- 35.Iverson LE, Rose JK. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477–484. 10.1016/0092-8674(81)90143-4 [DOI] [PubMed] [Google Scholar]
- 36.Bandi P, Pagliaccetti NE, Robek MD. 2010. Inhibition of type III interferon activity by orthopoxvirus immunomodulatory proteins. J. Interferon Cytokine Res. 30:123–134. 10.1089/jir.2009.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, Raveche E, Kotenko SV. 2006. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 66:4468–4477. 10.1158/0008-5472.CAN-05-3653 [DOI] [PubMed] [Google Scholar]
- 38.Amicone L, Spagnoli FM, Späth G, Giordano S, Tommasini C, Bernardini S, De Luca V, Della Rocca C, Weiss MC, Comoglio PM, Tripodi M. 1997. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO J. 16:495–503. 10.1093/emboj/16.3.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robek MD, Boyd BS, Chisari FV. 2005. Lambda interferon inhibits hepatitis B and C virus replication. J. Virol. 79:3851–3854. 10.1128/JVI.79.6.3851-3854.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263–275. 10.1016/S1535-6108(03)00241-1 [DOI] [PubMed] [Google Scholar]
- 41.Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, Lichty B, Power A, Johnston RN, Hamilton M, Parney I, Bell JC, Forsyth PA. 2006. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J. Natl. Cancer Inst. 98:1546–1557. 10.1093/jnci/djj413 [DOI] [PubMed] [Google Scholar]
- 42.Stewart JH, IV, Ahmed M, Northrup SA, Willingham M, Lyles DS. 2011. Vesicular stomatitis virus as a treatment for colorectal cancer. Cancer Gene Ther. 18:837–849. 10.1038/cgt.2011.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu L, Huang TG, Meseck M, Altomonte J, Ebert O, Shinozaki K, García-Sastre A, Fallon J, Mandeli J, Woo SL. 2008. rVSV(M Delta 51)-M3 is an effective and safe oncolytic virus for cancer therapy. Hum. Gene Ther. 19:635–647. 10.1089/hum.2007.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon ID, Publicover J, Rose JK. 2007. Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: vector spread correlates with induction of immune responses and persistence of genomic RNA. J. Virol. 81:2078–2082. 10.1128/JVI.02525-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trottier MD, Lyles DS, Reiss CS. 2007. Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J. Neurovirol. 13:433–445. 10.1080/13550280701460565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Pol AN, Dalton KP, Rose JK. 2002. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J. Virol. 76:1309–1327. 10.1128/JVI.76.3.1309-1327.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Pol AN, Ding S, Robek MD. 2014. Long-distance interferon signaling within the brain blocks virus spread. J. Virol. 88:3695–3704. 10.1128/JVI.03509-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forger JM, III, Bronson RT, Huang AS, Reiss CS. 1991. Murine infection by vesicular stomatitis virus: initial characterization of the H-2d system. J. Virol. 65:4950–4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbert SC. 2012. T-cell-inducing vaccines - what's the future. Immunology 135:19–26. 10.1111/j.1365-2567.2011.03517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, Weiner DB. 2009. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood 113:5868–5877. 10.1182/blood-2008-11-190520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrow MP, Yan J, Pankhong P, Ferraro B, Lewis MG, Khan AS, Sardesai NY, Weiner DB. 2010. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukin-12 (IL-12) or IL-28B vaccine adjuvants in rhesus macaques. Clin. Vaccine Immunol. 17:1493–1499. 10.1128/CVI.00181-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrow MP, Yan J, Pankhong P, Shedlock DJ, Lewis MG, Talbott K, Toporovski R, Khan AS, Sardesai NY, Weiner DB. 2010. IL-28B/IFN-lambda 3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol. Ther. 18:1714–1723. 10.1038/mt.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siebler J, Wirtz S, Weigmann B, Atreya I, Schmitt E, Kreft A, Galle PR, Neurath MF. 2007. IL-28A is a key regulator of T-cell-mediated liver injury via the T-box transcription factor T-bet. Gastroenterology 132:358–371. 10.1053/j.gastro.2006.10.028 [DOI] [PubMed] [Google Scholar]
- 54.Mennechet FJ, Uze G. 2006. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood 107:4417–4423. 10.1182/blood-2005-10-4129 [DOI] [PubMed] [Google Scholar]
- 55.Mueller RE, Muldoon RL, Jackson GG. 1969. Communicability of enteric live adenovirus type 4 vaccine in families. J. Infect. Dis. 119:60–66. 10.1093/infdis/119.1.60 [DOI] [PubMed] [Google Scholar]
- 56.Stanley ED, Jackson GG. 1969. Spread of enteric live adenovirus type 4 vaccine in married couples. J. Infect. Dis. 119:51–59. 10.1093/infdis/119.1.51 [DOI] [PubMed] [Google Scholar]
- 57.Lichtenstein DL, Wold WS. 2004. Experimental infections of humans with wild-type adenoviruses and with replication-competent adenovirus vectors: replication, safety, and transmission. Cancer Gene Ther. 11:819–829. 10.1038/sj.cgt.7700765 [DOI] [PubMed] [Google Scholar]
- 58.Schwartz AR, Togo Y, Hornick RB. 1974. Clinical evaluation of live, oral types 1, 2, and 5 adenovirus vaccines. Am. Rev. Respir. Dis. 109:233–239 [DOI] [PubMed] [Google Scholar]
- 59.Hehir KM, Armentano D, Cardoza LM, Choquette TL, Berthelette PB, White GA, Couture LA, Everton MB, Keegan J, Martin JM, Pratt DA, Smith MP, Smith AE, Wadsworth SC. 1996. Molecular characterization of replication-competent variants of adenovirus vectors and genome modifications to prevent their occurrence. J. Virol. 70:8459–8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lochmüller H, Jani A, Huard J, Prescott S, Simoneau M, Massie B, Karpati G, Acsadi G. 1994. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (delta E1 + delta E3) during multiple passages in 293 cells. Hum. Gene Ther. 5:1485–1491. 10.1089/hum.1994.5.12-1485 [DOI] [PubMed] [Google Scholar]
- 61.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. 2006. Antitumor activity of IFN-lambda in murine tumor models. J. Immunol. 176:7686–7694. 10.4049/jimmunol.176.12.7686 [DOI] [PubMed] [Google Scholar]
- 62.Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, de la Torre A, Lasfar A, Kotenko SV. 2010. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol. Immunother. 59:1059–1071. 10.1007/s00262-010-0831-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Numasaki M, Tagawa M, Iwata F, Suzuki T, Nakamura A, Okada M, Iwakura Y, Aiba S, Yamaya M. 2007. IL-28 elicits antitumor responses against murine fibrosarcoma. J. Immunol. 178:5086–5098. 10.4049/jimmunol.178.8.5086 [DOI] [PubMed] [Google Scholar]