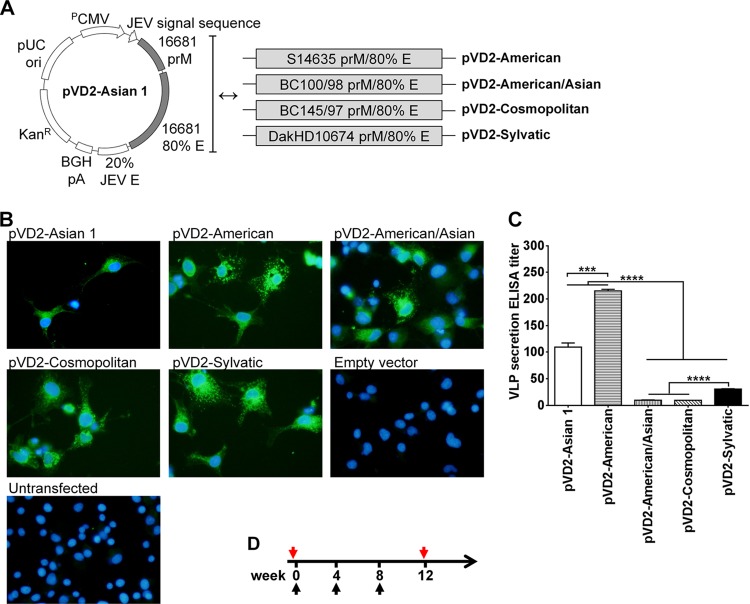
FIG 2.
Vaccine design, analysis of recombinant antigen expression and secretion, and immunization schedule. (A) Schematic representation of plasmid vectors containing DNA of the different DENV-2 genotypes: pVD2-Asian 1, pVD2-American, pVD2-American/Asian, pVD2-Cosmopolitan, and pVD2-Sylvatic. These plasmid constructs include the human cytomegalovirus early gene promoter (PCMV), the JEV signal sequence, prM, 80% of the E-gene region (amino acids 1 to 396) of each DENV-2 genotype, 20% of the homologous E-gene region of JEV (amino acids 397 to 495), and a bovine growth hormone poly(A) signal (BGH pA). (B) Immunofluorescence analysis of recombinant protein expression in COS-1 cells transfected with plasmids containing DNA of the different DENV-2 genotypes. After fixation and permeabilization, recombinant proteins were detected with anti-DENV-2 MHIAF, followed by incubation with goat anti-mouse IgG-fluorescein isothiocyanate and DAPI to counterstain the nucleus. The characteristic green fluorescence in the cells indicates positivity for the intracellular expression of recombinant proteins. (C) Detection and quantification by an Ag-capture ELISA of secreted VLPs in culture supernatants harvested from transiently transformed COS-1 cells. Data are presented as means ± SEMs of two independent repeat transfection experiments. Means were analyzed by one-way ANOVA (P < 0.05), and Holm-Sidak's posttest significance is indicated with asterisks (***, P = 0.0002; ****, P < 0.0001). (D) Schedule of immunization with different plasmid vectors containing DNA of the different DENV-2 genotypes. Groups of 4-week-old BALB/c mice were immunized with three doses of 100 μg DNA intramuscularly. At 4 weeks following the last immunization, blood samples from each group of mice were taken and pooled for further serum antibody analyses. Black arrows, immunization times; red arrows, blood sampling times.