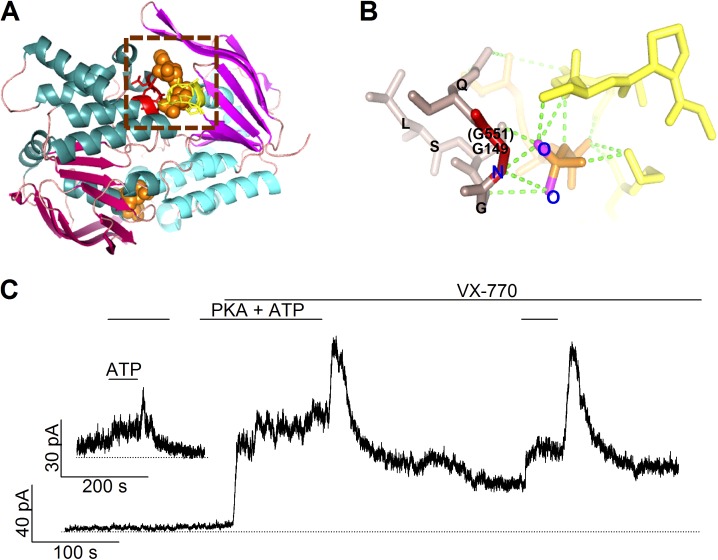
Figure 1.
The roles of the conserved glycine at position 551 in the structure and function of CFTR. (A) The structure of a prototypical NBD dimer. A ribbon representation of the model was generated with PyMOL by using the dimeric MJ0796 NBD structure (PDB ID: 1L2T). The head and tail subdomains of each NBD are shown in magenta and cyan, respectively. Two ATP molecules, shown in a space-filling model, are sandwiched in the dimer interface. One of the signature sequences is shown in red, with the Walker A and B motifs of the partner NBD in yellow. (B) An expanded view of the part enclosed by the dashed square in A showing the hydrogen bond network between ATP and the signature sequence. The G149 residue (i.e., G551 in CFTR’s NBD1) is highlighted in red, the rest of the signature sequence is in brown. ATP is presented in orange, and green dotted lines show possible hydrogen bonds. Parts of the Walker A and B motifs from the partner NBD are depicted in yellow. (C) A real-time current trace of G551D-CFTR channels showing an effect of VX-770 in potentiating the channel activity as well as a biphasic response of the current to ATP washout. The inset shows an experiment in which PKA was removed long before the addition and removal of ATP.