Abstract
BACKGROUND
Neonatal-onset multisystem inflammatory disease is characterized by fever, urticarial rash, aseptic meningitis, deforming arthropathy, hearing loss, and mental retardation. Many patients have mutations in the cold-induced autoinflammatory syndrome 1 (CIAS1) gene, encoding cryopyrin, a protein that regulates inflammation.
METHODS
We selected 18 patients with neonatal-onset multisystem inflammatory disease (12 with identifiable CIAS1 mutations) to receive anakinra, an interleukin-1–receptor antagonist (1 to 2 mg per kilogram of body weight per day subcutaneously). In 11 patients, anakinra was withdrawn at three months until a flare occurred. The primary end points included changes in scores in a daily diary of symptoms, serum levels of amyloid A and C-reactive protein, and the erythrocyte sedimentation rate from baseline to month 3 and from month 3 until a disease flare.
RESULTS
All 18 patients had a rapid response to anakinra, with disappearance of rash. Diary scores improved (P<0.001) and serum amyloid A (from a median of 174 mg to 8 mg per liter), C-reactive protein (from a median of 5.29 mg to 0.34 mg per deciliter), and the erythrocyte sedimentation rate decreased at month 3 (all P<0.001), and remained low at month 6. Magnetic resonance imaging showed improvement in cochlear and leptomeningeal lesions as compared with baseline. Withdrawal of anakinra uniformly resulted in relapse within days; retreatment led to rapid improvement. There were no drug-related serious adverse events.
CONCLUSIONS
Daily injections of anakinra markedly improved clinical and laboratory manifestations in patients with neonatal-onset multisystem inflammatory disease, with or without CIAS1 mutations. (ClinicalTrials.gov number, NCT00069329.)
Neonatal-onset multisystem inflammatory disease (NOMID), also known as chronic infantile neurologic cutaneous articular (CINCA) syndrome, is a rare chronic inflammatory disease.1,2 An urticaria-like rash develops within the first six weeks of life, and a characteristic bony overgrowth predominantly involving the knees develops in most affected children. Central nervous system (CNS) manifestations include chronic aseptic meningitis, increased intracranial pressure, cerebral atrophy, ventriculomegaly, and chronic papilledema, with associated optic-nerve atrophy and loss of vision, mental retardation, seizures, and sensorineural hearing loss. Other manifestations include short stature, hepatosplenomegaly, leukocytosis, and an elevation in serum levels of amyloid A and C-reactive protein and in the erythrocyte sedimentation rate. Therapies are aimed at suppressing inflammation and have included high-dose corticosteroids, disease-modifying antirheumatic drugs, and biologic agents targeting tumor necrosis factor (TNF). Although these medications are moderately effective, inflammation persists in most children, and a 20 percent mortality rate has been reported before adulthood.3
The discovery of the genetic basis of neonatal-onset multisystem inflammatory disease4,5 has led to the inclusion of this syndrome in a group of hereditary systemic autoinflammatory disorders.6 Mutations in the gene for the cold-induced autoinflammatory syndrome 1 (CIAS1), mostly newly occurring ones, are present in about 60 percent of children who receive a clinical diagnosis of the disease. Patients with and those without CIAS1 mutations have similar disease phenotypes.5 CIAS1 mutations were initially identified in two phenotypically milder familial syndromes,7 familial cold autoinflammatory syndrome2 and the Muckle–Wells syndrome.2 Both disorders are characterized by episodes of urticarial rash and systemic inflammation but not bony overgrowth, chronic meningitis, or mental retardation.
CIAS1 encodes cryopyrin (also known as NALP3),8 which belongs to a group of interacting proteins that form a macromolecular complex termed the “inflammasome.”8 Inflammasome assembly leads to the activation of caspase 1, which cleaves pro–interleukin-1β into its bioactive form (Fig. 1 in the Supplementary Appendix, available with the full text of this article at www.nejm.org). There is conflicting evidence as to whether cryopyrin activates nuclear factor- κB (NF-κB), another mediator of inflammation.9–15 Selective blockade of interleukin-1β permits a stringent in vivo test of the relative contributions of interleukin-1β–dependent pathways and interleukin-1β–independent pathways in the pathophysiology and organ-specific manifestations of neonatal-onset multisystem inflammatory disease, in particular the CNS manifestations of the disease.
Isolated case reports have suggested that as an interleukin-1–receptor antagonist, anakinra may be effective in the treatment of rash and the constitutional symptoms of neonatal-onset multisystem inflammatory disease.16–18 We systematically assessed the effect of anakinra on a broader range of disease manifestations, including ones that affect the CNS, in a cohort of patients with neonatal-onset multisystem inflammatory disease who were seen at one center.
METHODS
PATIENTS
We selected patients between the ages of 4 and 32 years who presented with at least two of the following clinical manifestations: urticarial rash, CNS involvement (e.g., papilledema, pleocytosis in the cerebrospinal fluid, and sensorineural hearing loss), or epiphyseal or patellar overgrowth on radiography. All patients had active disease despite treatment with nonsteroidal antiinflammatory drugs and disease-modifying antirheumatic drugs or corticosteroids. Two patients who were receiving etanercept completed a 21-day washout period before beginning treatment with anakinra.
STUDY DESIGN AND TREATMENT
The study protocol was approved by the institutional review board at the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases. All patients or their parents or legal guardians provided written informed consent. Between September 2003 and July 2004, 20 patients were screened. Of those patients, 18 from 16 referring sites were enrolled (the 2 patients who were excluded had neither CNS involvement nor bone disease). Anakinra (Kineret, Amgen), which was procured commercially by the National Institutes of Health (NIH) Clinical Center Pharmacy, was started at a dose of 1 mg per kilogram of body weight per day by subcutaneous injection and was increased to a maximum of 2 mg per kilogram per day if clinical disease persisted or laboratory measures remained abnormal. Efficacy assessments were made at the NIH at one, three, and six months. At three months, patients who had a response to treatment underwent an inpatient withdrawal period until they fulfilled predefined criteria for a clinical flare (defined as at least two of the following criteria: an increase in the rash score for four days, a temperature >37°C [98.6°F] on four or more occasions, vomiting or headache for three days, or a worsening of any neurosensory symptom) or for a maximum of seven days.
If a flare of the disease occurred, anakinra therapy was resumed, and patients entered the ongoing extension period of the study (up to 24 months). Because of the severity of the flares — which included pericarditis in 1 patient, corneal infiltrates in 3 patients, and uveitis in 2 patients — and the significance of the study findings in the first 11 patients, the NIH bioethics committee recommended the discontinuation of the withdrawal phase.
PRIMARY END POINTS
The primary end points included a change in a disease-specific daily diary score, changes in the acute-phase reactants (serum amyloid A, C-reactive protein, and the erythrocyte sedimentation rate) from baseline to three months and from three months until a flare in the disease occurred. The diary included daily reports of fever, rash, headache, joint pain, and vomiting, which were rated on a scale of 0 to 4 for increasing severity of each of the five symptoms (possible range, 0 to 20). Diary data were collected for three consecutive weeks, and serum levels of amyloid A and C-reactive protein and the erythrocyte sedimentation rate were measured on two to four occasions before anakinra treatment was started. The level of C-reactive protein and the erythrocyte sedimentation rate were determined at the NIH; the level of serum amyloid A was measured as previously reported.19
SECONDARY END POINTS
Childhood health assessment questionnaires, audiography, and vision evaluations were performed at baseline and at follow-up at one, three, and six months. All patients underwent a lumbar puncture at baseline and at three months. Magnetic resonance imaging (MRI) of the brain with gadolinium-enhanced fluid-attenuated inversion recovery (FLAIR) sequences of the inner ear and fast imaging employing steady-state acquisition (FIESTA) (involving 15 patients) and an MRI of the worse knee were performed at baseline and at three months. Among 17 English-speaking patients, cognitive function was assessed with the use of the following age-appropriate standardized tests: the Wechsler Preschool and Primary Scale of Intelligence — Third Edition (administered to 4 patients), the Wechsler Intelligence Scale for Children — Fourth Edition (to 8 patients), the Wechsler Adult Intelligence Scale — Third Edition (to 3 patients), and the Vineland Adaptive Behavior Scales — Interview Edition (to 2 patients).
Other end points included an analysis of drug safety; remission of inflammation (defined by a serum amyloid A level below 10 mg per liter, a C-reactive protein level below 0.5 mg per deciliter, an erythrocyte sedimentation rate below 20 mm per hour, and a daily diary score below 0.5); changes in brain MRI, as read by one radiologist who was unaware of patients’ treatment assignments; corticosteroid dose; and changes in the levels of proinflammatory and antiinflammatory cytokines (including endogenous interleukin-1–receptor antagonist [interleukin-1Ra] and recombinant interleukin-1–receptor antagonist [anakinra]) in serum and cerebrospinal fluid, chemokines and endothelial markers (Pierce Biotechnology), and the pharmacokinetic profile. Spontaneous and stimulated secretions of interleukin-1β were measured in culture supernatants from peripheral-blood mononuclear cells cultured for 24 hours in the presence and absence of lipopolysaccharide (2 μg per milliliter). Transcriptional analysis was performed from whole-blood samples as previously described.20 Control blood samples were obtained from 25 anonymous healthy donors and from 10 of the patients’ parents, all with consent for this purpose.
STATISTICAL ANALYSIS
The study was designed to have a statistical power of 80 percent with the use of a two-sided test, with a level of significance of 0.05, to detect a mean difference in diary scores before and after treatment equal in magnitude to the standard deviations of the differences. Differences were tested with the use of two-sided tests, the Wilcoxon signed-rank test, or the Wilcoxon rank-sum test, for nonparametric data at a significance level of 0.05.
RESULTS
All 18 patients had active disease, as indicated by the diary scores and the results of the clinical and laboratory examination; 12 (67 percent) had mutations in exon 3 of CIAS1. Acute-phase reactants were elevated at baseline despite treatment with immunomodulatory medications and corticosteroids (Tables 1 and 2). All enrolled patients had clinical CNS disease. Of the patients with a nontraumatic lumbar puncture, the majority had increased intracranial pressure and pleocytosis (a white-cell count above 6 cells per cubic millimeter). Other clinical findings included urticarial rash, papilledema, conjunctivitis, uveitis, hearing loss, and bony overgrowth (Fig. 1A and 1C, and Fig. 2 of the Supplementary Appendix). Most patients had heights below the third percentile (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of the 18 Patients.*
| Characteristic | Value |
|---|---|
| Demographic | |
| Age — yr | 11.0±4.4 |
|
| |
| Age group — no. (%) | |
|
| |
| 4–8 yr | 7 (39) |
|
| |
| 9–12 yr | 6 (33) |
|
| |
| 13–18 yr | 2 (11) |
|
| |
| ≥18 yr | 3 (17) |
|
| |
| Sex — no. (%) | |
|
| |
| Female | 8 (44) |
|
| |
| Male | 10 (56) |
|
| |
| Race or ethnic group — no. (%)† | |
|
| |
| White | 11 (61) |
|
| |
| Black | 1 (6) |
|
| |
| Hispanic | 4 (22) |
|
| |
| Asian | 1 (6) |
|
| |
| Native American | 1 (6) |
|
| |
| Clinical | |
|
| |
| Mutation in exon 3 of CIAS1 — no. (%) | 12 (67) |
|
| |
| DMARDs — no. (%) | |
|
| |
| Methotrexate | 9 (50) |
|
| |
| Etanercept | 3 (17) |
|
| |
| Thalidomide | 1 (6) |
|
| |
| Colchicine | 2 (11) |
|
| |
| Oral corticosteroids — no. (%) | 11 (61) |
|
| |
| Oral corticosteroid dose — mg/kg/day | 0.85±0.7 |
|
| |
| NSAIDs — no. (%) | 12 (67) |
|
| |
| Clinical | |
| Clinical manifestations — no. (%) | |
|
| |
| Papilledema | 13 (72) |
|
| |
| Stroke | 4 (22) |
|
| |
| Seizures | 3 (17) |
|
| |
| Increased intracranial pressure (>180 mm of water) — no. (%)‡ | 13 (93) |
|
| |
| Aseptic meningitis (white-cell count, >6 cells/mm3) — no. (%)§ | 12 (80) |
|
| |
| Cognitive function (IQ) — no. (%)¶ | |
|
| |
| Extremely low (<70) | 6 (35) |
|
| |
| Borderline (70–79) | 2 (12) |
|
| |
| Low average (80–89) | 4 (24) |
|
| |
| Average (90–109) | 4 (24) |
|
| |
| High average (110–119) | 0 |
|
| |
| Superior (120–129) | 1 (6) |
|
| |
| Growth retardation (3rd percentile) — no. (%) | 14 (78) |
|
| |
| Bony overgrowth — no. (%) | 11 (61) |
|
| |
| Hearing loss — no. (%) | 15 (83) |
|
| |
| Normal (−10 to 20 dB) | 3 (17) |
|
| |
| Mild (>20 to ≤40 dB) | 4 (22) |
|
| |
| Moderate (>40 to ≤70 dB) | 5 (28) |
|
| |
| Severe (>70 to <95 dB) | 4 (22) |
|
| |
| Profound (≥95 dB) | 2 (11) |
|
| |
| Urticarial rash — no. (%) | 17 (94) |
|
| |
| Baseline abnormalities on brain MRI — no. (%)|| | |
|
| |
| Leptomeningeal enhancement | 8 (44) |
|
| |
| Dural enhancement | 5 (28) |
|
| |
| Ventriculomegaly** | 8 (44) |
|
| |
| Cochlear enhancement | 17 (94) |
|
| |
| Arachnoid adhesions†† | 10 (67) |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. DMARDs denotes disease-modifying antirheumatic drugs, and NSAIDs nonsteroidal antiinflammatory drugs.
Race was self-reported by the patient.
Intracranial pressures were obtained for 14 patients at baseline.
Cerebrospinal-fluid cell counts were obtained for 15 patients at baseline.
Cognitive function was assessed with the use of the following age-appropriate standardized tests among 17 English-speaking patients: Wechsler Preschool and Primary Scale of Intelligence — Third Edition (4 patients), Wechsler Intelligence Scale for Children — Fourth Edition (8 patients), Wechsler Adult Intelligence Scale — Third Edition (3 patients), and the Vineland Adaptive Behavior Scales — Interview Edition (2 patients).
All patients had at least one abnormality on MRI.
Two additional patients had ventriculoperitoneal shunts.
Fifteen patients had FIESTA sequences.
Table 2.
Measures of Disease Activity and Improvement from Baseline.*
| Measure | Phase of Open-Label Treatment | P Value† | P Value‡ | |||
|---|---|---|---|---|---|---|
| Baseline | One Month | Three Months | Six Months | |||
| Primary measure of clinical response | ||||||
|
| ||||||
| Global diary score§ | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 3.70 | 0.79 | 0.29 | 0.26 | ||
|
| ||||||
| Interquartile range | 2.16–4.84 | 0.26–1.25 | 0.08–0.84 | 0.12–0.70 | ||
|
| ||||||
| Primary measures of laboratory response | ||||||
|
| ||||||
| Erythrocyte sedimentation rate (mm/hr) | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 57.5 | 12.5 | 18.0 | 16.0 | ||
|
| ||||||
| Interquartile range | 35.0–73.0 | 11.0–24.0 | 9.0–25.0 | 11.0–29.0 | ||
|
| ||||||
| C-reactive protein (mg/dl) | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 5.29 | 0.93 | 0.34 | 0.40 | ||
|
| ||||||
| Interquartile range | 4.00–10.50 | 0.49–1.94 | 0.16–0.89 | 0.10–0.91 | ||
|
| ||||||
| Serum amyloid A (mg/liter) | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 174 | 25 | 8 | 6 | ||
|
| ||||||
| Interquartile range | 131–436 | 9–97 | 3–34 | 3–16 | ||
|
| ||||||
| Secondary measures of clinical response | ||||||
|
| ||||||
| CHAQ score¶ | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 1.30 | 0.64 | 0.37 | 0.34 | ||
|
| ||||||
| Interquartile range | 0.65–1.57 | 0.31–1.03 | 0.12–0.72 | 0.13–0.68 | ||
|
| ||||||
| Physician’s global assessment (mm)|| | 0.001 | <0.001 | ||||
|
| ||||||
| Median | 16.5 | 9.0 | 4.5 | 4.5 | ||
|
| ||||||
| Interquartile range | 8.0–32.0 | 7.0–14.0 | 4.0–10.0 | 2.0–8.0 | ||
|
| ||||||
| Parent’s global assessment (mm)|| | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 48.5 | 10.0 | 5.5 | 5.5 | ||
|
| ||||||
| Interquartile range | 23.5–52.0 | 4.0–28.0 | 2.0–16.0 | 2.0–8.5 | ||
|
| ||||||
| Visual-analogue scale for pain (mm)|| | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 38.0 | 12.0 | 3.0 | 5.5 | ||
|
| ||||||
| Interquartile range | 22.0–60.0 | 6.0–20.0 | 2.0–10.0 | 2.0–13.0 | ||
|
| ||||||
| Dose of prednisone or prednisone equivalent dose (mg/kg/day)** | 0.002 | 0.001 | ||||
|
| ||||||
| Median | 0.46 | ND | 0.30 | 0.17 | ||
|
| ||||||
| Interquartile range | 0.21–0.96 | 0.20–0.38 | 0.08–0.24 | |||
|
| ||||||
| Secondary measures of laboratory response | ||||||
|
| ||||||
| White-cell count (×10−3/mm3) | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 17.2 | 8.9 | 9.3 | 8.4 | ||
|
| ||||||
| Interquartile range | 13.6–21.5 | 7.1–13.9 | 7.5–11.2 | 6.8–12.1 | ||
|
| ||||||
| Absolute neutrophil count (×10−3/mm3) | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 12.4 | 5.0 | 5.4 | 5.1 | ||
|
| ||||||
| Interquartile range | 9.9–15.5 | 3.9–9.8 | 3.0–7.4 | 2.8–7.2 | ||
|
| ||||||
| Hemoglobin (g/dl) | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 11.2 | 12.5 | 13.3 | 13.4 | ||
|
| ||||||
| Interquartile range | 10.4–11.8 | 12.0–13.1 | 12.5–14.4 | 12.4–14.1 | ||
|
| ||||||
| Platelets (×10−3/mm3) | <0.001 | <0.001 | ||||
|
| ||||||
| Median | 423 | 326 | 302 | 296 | ||
|
| ||||||
| Interquartile range | 380–531 | 249–417 | 219–368 | 269–409 | ||
|
| ||||||
| Height (cm)†† | 112.9±24.8 | 113.3±24.6 | 115.0±25.1 | 116.0±25.2 | <0.001 | <0.001 |
|
| ||||||
| Weight (kg) | 28.7±15.8 | 30.0±16.2 | 31.4±17.3 | 32.7±18.3 | <0.001 | 0.001 |
|
| ||||||
| Cerebrospinal fluid pressure (mm of water)‡‡ | <0.001 | NA | ||||
|
| ||||||
| Median | 287 | ND | 197 | ND | ||
|
| ||||||
| Interquartile range | 250–325 | ND | 167–222 | ND | ||
|
| ||||||
| Cerebrospinal fluid protein (mg/dl)§§ | 0.05 | NA | ||||
|
| ||||||
| Median | 35 | ND | 33 | ND | ||
|
| ||||||
| Interquartile range | 24–51 | ND | 23–40 | ND | ||
|
| ||||||
| White-cell count in cerebrospinal fluid (cells/mm3)§§ | 0.05 | NA | ||||
|
| ||||||
| Median | 19 | ND | 9 | ND | ||
|
| ||||||
| Interquartile range | 6–49 | ND | 6–12 | ND | ||
|
| ||||||
| Neutrophil count in cerebrospinal fluid (cells/mm3)§§ | 0.04 | NA | ||||
|
| ||||||
| Median | 10.2 | ND | 3.8 | ND | ||
|
| ||||||
| Interquartile range | 4.0–25.5 | ND | 1.8–6.7 | ND | ||
Plus–minus values are means ±SD. NA denotes not applicable, and ND not done.
P values are for the comparison of baseline values with values obtained at three months.
P values are for the comparison of baseline values with values obtained at six months.
Median daily scores of five symptoms (fever, rash, headache, joint pain, and vomiting) were evaluated daily with the use of a scale that ranged from 0 (no symptoms) to 4 (severe symptoms) (possible total range, 0 to 20). The maximal daily score measured was 14; the minimal score was 0.
Scores for the Childhood Health Assessment Questionnaire (CHAQ), a standardized test for the assessment of disability, range from 0 to 3, with higher scores indicating more severe impairment.
A visual-analogue scale was used in which a value of 100 mm indicates the worst possible measure for the condition assessed by the test.
Values are for 11 patients who were receiving corticosteroids at study entry.
Values are for 15 patients with open growth plates only. At six months, the growth velocity in percentile was 74; the heights of 12 of these patients fell below the 3rd percentile for age at the beginning of the study.
Cerebrospinal fluid pressures could be evaluated in 12 patients (i.e., could be obtained on both visits, and patients did not cry during the procedure).
Cerebrospinal fluid could be evaluated in 14 patients (i.e., could be obtained on both visits, and patients had a red-cell count of less than 50 cells per cubic millimeter in the cerebrospinal fluid).
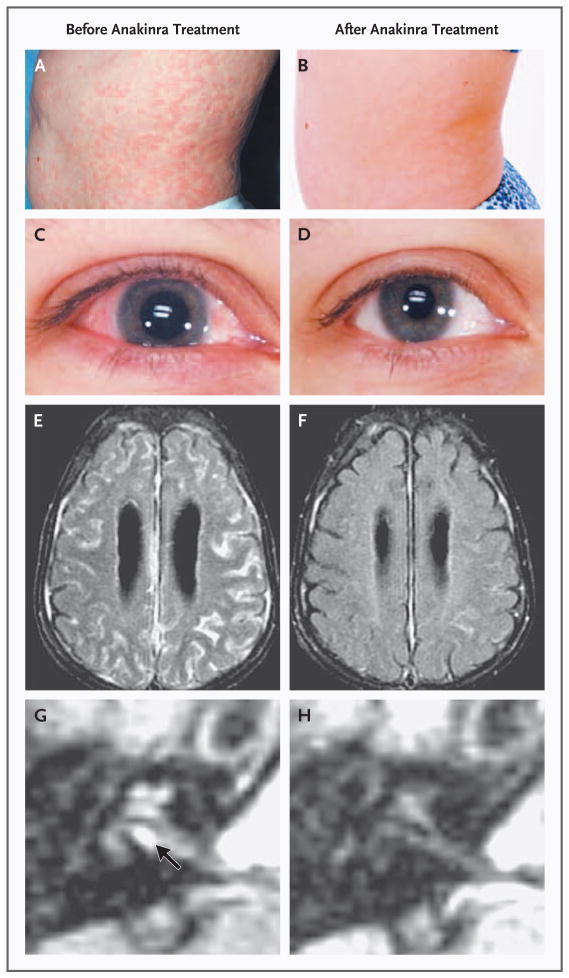
Figure 1. Inflammatory Organ Manifestations in Neonatal-Onset Multisystem Inflammatory Disease before (Panels A, C, E, and G) and after (Panels B, D, F, and H) Treatment with Anakinra.
The severity of rash, conjunctivitis, and leptomeningeal and cochlear enhancement on MRI is shown at baseline (Panels A, C, E, and G [arrow], respectively) and after three months (Panels B, D, F, and H) of anakinra therapy.
Unenhanced MRI scans showed ventriculomegaly in eight patients and mild-to-moderate cerebral atrophy in three patients. Two patients had ventriculoperitoneal shunts. High-resolution FIESTA images showed arachnoid adhesions (Fig. 2E in the Supplementary Appendix). FLAIR sequences performed after the administration of contrast material were used to visualize potential inflammatory CNS lesions. Leptomeningeal enhancement was detected in 8 patients, and abnormal cochlear enhancement was detected in 17 patients (Table 1 and Fig. 1E and 1G). Patients with leptomeningeal or dural enhancement had significantly lower IQ levels than did patients without enhancement (median values of 66 and 89, respectively; P = 0.03), and median protein levels in the cerebrospinal fluid were 52 mg per deciliter and 34 mg per deciliter, respectively (P = 0.07).
EFFECTS OF ANAKINRA
All 18 patients had an immediate clinical response to anakinra. Rash and conjunctivitis disappeared within three days in all cases (Fig. 1A, 1B, 1C, and 1D). The diary scores significantly decreased at three months. Levels of serum amyloid A and C-reactive protein and the erythrocyte sedimentation rate all fell significantly with treatment in all patients (Table 2).
After three months of treatment, 11 patients underwent an inpatient withdrawal period for a maximum of seven days. All but one patient fulfilled prespecified criteria for a flare of disease. The one patient who did not fulfill the criteria had six days of rash, one episode of fever, and three days of joint pain and conjunctivitis. The median time until a flare of the disease occurred was 5 days (range, 2.5 to 7) (Fig. 3 of the Supplementary Appendix). Patients had a response promptly after resuming anakinra, and improvements were sustained at the six-month follow-up evaluation (Table 2).
At six months, six patients (33 percent) showed improved hearing on audiography, and nine patients (50 percent) had stable hearing, relative to baseline (Table 1 and Fig. 4 of the Supplementary Appendix). The hearing of one patient improved at high frequencies and deteriorated at low frequencies. Vision remained stable in all patients, and pain, global assessments by parents and physicians, and scores on the Childhood Health Assessment Questionnaire improved significantly (Table 2). The median dose of prednisone was significantly lower at three and six months than at baseline (Table 2). Remission of inflammatory symptoms occurred in 8 of 18 patients (44 percent) at three months and in 10 of 18 patients (56 percent) at six months.
CNS RESPONSE TO TREATMENT
All patients had headache at baseline. During therapy, median daily headache scores (rated from 0 to 4 for increasing severity) decreased from 0.5 to 0.1 (P<0.001). In eight patients, headache completely resolved at three months. In 12 patients for whom cerebrospinal fluid could be evaluated, intracranial pressures, protein levels, and white-cell counts also decreased significantly (Table 2). In the cerebrospinal fluid, white-cell counts correlated with interleukin-6 levels (correlation coefficient, 0.63; P = 0.006). Headache recurred or worsened promptly in all patients during the flare period, with a median headache score of 0.8 (P = 0.007 for the comparison with the score at three months after the initiation of treatment). Of the 17 patients with cochlear enhancement on initial MRI (Fig. 1G), 13 showed a decrease in or disappearance of cochlear enhancement (Fig 1H), 1 had an increased level, and 3 remained unchanged after three months of therapy. In addition, leptomeningeal enhancement, which was present in eight patients before drug treatment, improved in all patients at three months (Fig. 1E and 1F).
CHANGES IN CYTOKINES WITH TREATMENT
Levels of interleukin-6 in serum and cerebrospinal fluid decreased with treatment and again increased in the serum when the drug was withheld (Table 3). TNF, E-selectin (a marker of endothelial activation), and the chemokine stromal-cell–derived factor 1 (SDF-1) also decreased with therapy. Levels of anakinra in the cerebrospinal fluid increased during therapy (P<0.001), suggesting drug penetration into the cerebrospinal fluid (Table 3).
Table 3.
Mean Cytokine and Chemokine Levels at Baseline, at Three Months, and during a Disease Flare.*
| Cytokine or Chemokine Analytes | Baseline | 3 Mo | P Value† | Flare | P Value‡ |
|---|---|---|---|---|---|
| Interleukin-6 in serum (pg/ml) | 0.01 | 0.008 | |||
| Median | 5.70 | 3.96 | 20.73 | ||
| Interquartile range | 3.19–15.97 | 1.90–5.50 | 4.90–31.08 | ||
| Interleukin-6 in cerebrospinal fluid | 0.04 | NA | |||
| Median | 43.93 | 21.61 | ND | ||
| Interquartile range | 26.19–93.37 | 7.76–68.90 | ND | ||
| TNF (pg/ml) | 0.006 | 0.5 | |||
| Median | 556 | 318 | 403 | ||
| Interquartile range | 83–646 | 81–452 | 361–488 | ||
| TNF receptor (pg/ml) | 0.008 | 0.04 | |||
| Median | 1154 | 650 | 889 | ||
| Interquartile range | 638–1459 | 412–1377 | 582–1215 | ||
| Stromal-cell–derived factor 1 (pg/ml) | 0.002 | 0.3 | |||
| Median | 1125 | 875 | 962 | ||
| Interquartile range | 385–2948 | 379–1111 | 349–1199 | ||
| E-selectin (ng/ml) | 0.002 | 0.2 | |||
| Median | 134 | 45 | 88 | ||
| Interquartile range | 80–196 | 41–61 | 43–105 | ||
| Interleukin-1–receptor antagonist in serum (pg/ml) | <0.001 | 0.001 | |||
| Median | 364 | 43,237 | 466 | ||
| Interquartile range | 232–1255 | 8795–200,300 | 208–763 | ||
| Interleukin-1–receptor antagonist in cerebrospinal fluid (pg/ml) | <0.001 | NA | |||
| Median | 211 | 1,136 | ND | ||
| Interquartile range | 77–352 | 497–1686 |
ND denotes not done, and NA denotes not applicable.
P values are for the comparison of baseline values with values obtained at three months.
P values are for the comparison of values at three months with values obtained during a disease flare at two to seven days after withdrawal of anakinra.
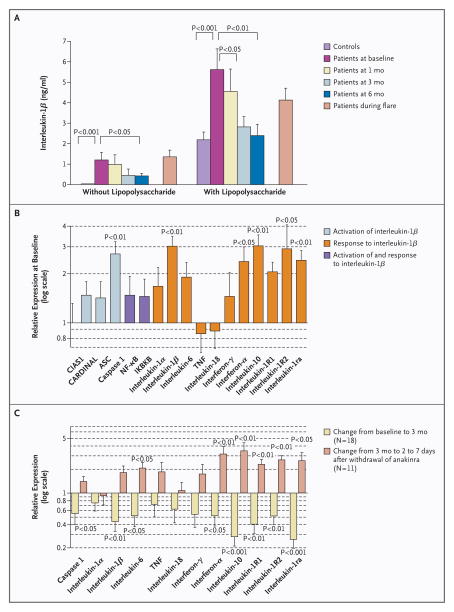
The patients’ cultured peripheral-blood mononuclear cells spontaneously secreted high levels of interleukin-1β, as compared with barely detectable levels in healthy controls, and had an exaggerated interleukin-1β response to lipopolysaccharide stimulation. Spontaneous and stimulated secretions of interleukin-1β decreased progressively with up to six months of therapy (Fig. 2A). Before treatment, transcript levels of several genes encoding proteins regulated by interleukin-1β were significantly increased, as compared with controls, whereas transcript levels of the mutant CIAS1 and levels of TNF and interleukin-18 were not increased (Fig. 2B). Anakinra decreased the expression of interleukin-1β and genes downstream of interleukin-1β, whereas such expression increased during anakinra withdrawal (Fig. 2C).
Figure 2. Mean (±SE) Serologic and Cellular Responses to Treatment.
Panel A shows levels of interleukin-1β in supernatants of cultures of peripheral-blood mononuclear cells (106 cells per milliliter), cultured for 24 hours with and without lipopolysaccharide (final concentration, 2 μg per milliliter), control subjects and patients at baseline; at one month, three months, and six months; and during a flare in the disease, during which time therapy with anakinra was intentionally withheld. Panels B and C show quantitative reverse-transcriptase–polymerase-chain-reaction analysis of gene products that are involved in the regulation of interleukin-1β activation, including CIAS1 encoding cryopyrin and genes encoding activation and recruitment domain (CARD) inhibitor of NF-κB–activating ligand (CARDINAL), apoptosis-associated speck-like protein with a CARD (ASC), and caspase 1; and molecules involved in the downstream response to interleukin-1β — interleukin 1α, 1β, 6, 18, and 10; TNF-α; interferon-γ and interferon-α; interleukin-1 receptor 1 and 2; and interleukin-1–receptor antagonist. NF-κB and inhibitor of kappa light polypeptide gene enhancer in B cells, kinase B (IKBKB) can be involved in both the regulation of and response to interleukin-1β. In Panel B, the level of expression of gene products in blood samples from the patients is expressed on a log (base-10) scale relative to the level of expression of gene products in blood samples from control subjects (assigned a value of 1) at baseline. Panel C shows the changes in the level of expression of gene products in blood samples from the patients from baseline to month 3 (in 18 patients) and from month 3 until two to seven days after the withdrawal of anakinra (in 11 patients).
There were no significant differences between patients with CIAS1 mutations and those without CIAS1 mutations in baseline clinical manifestations or response to anakinra. However, this study was not powered to detect such differences.
SAFETY AND TOLERABILITY
None of the patients discontinued drug treatment. A localized, erythematous, and sometimes painful skin reaction at the injection site developed in eight patients (44 percent) and had disappeared in all patients at six weeks. Adverse events during treatment included upper respiratory infections (in 15 patients), urinary tract infections (in 2), and a hospital admission for dehydration from nonbacterial diarrhea (in 1).
DISCUSSION
We found that anakinra, an interleukin-1 antagonist, significantly decreased the major organ manifestations in patients with neonatal-onset multisystem inflammatory disease. Rash and measures of inflammation rapidly improved with treatment, worsened with drug withdrawal, and promptly responded to the reinitiation of therapy. Elevations in intracranial pressure and in cerebrospinal fluid protein also decreased with therapy, and hearing improved or stabilized in most patients. These findings suggest that peripheral, as well as CNS, manifestations of this disease are driven by interleukin-1β and will benefit from the systemic administration of anakinra. These data define the clinical and molecular phenotype of neonatal-onset multisystem inflammatory disease as induced by interleukin-1β excess.
The identification of CIAS1 mutations in neonatal-onset multisystem inflammatory disease, familial cold autoinflammatory syndrome, and the Muckle–Wells syndrome has led to the notions that these diseases are part of a disease spectrum, with familial cold autoinflammatory syndrome at the mildest end of the symptom spectrum and neonatal-onset multisystem inflammatory disease at the most severe end. Factors determining the phenotype of the disease include the type of mutation and the patient’s genetic background.21 Previous isolated case reports in patients with the range of CIAS1-associated diseases16–18,22,23 described responses of constitutional symptoms, urticarial rash, and acute-phase reactants to anakinra, but a systematic analysis of the effect of anakinra on CNS manifestations, hearing and vision loss, or joint disease has been lacking.
Given the rarity of neonatal-onset multisystem inflammatory disease, limitations of our study necessarily include its small size and the lack of a randomized, placebo-controlled design, and a follow-up of six months. Nevertheless, the magnitude of the clinical responses that were observed, the incorporation of an inpatient withdrawal phase to induce a disease flare, and the detailed analysis of organ-specific disease manifestations (including blinded evaluation of MRI studies) provide evidence of important clinical benefits derived from interleukin-1 blockade in this condition.
We used highly sensitive MRI sequences to identify enhancing CNS lesions in the leptomeninges, dura, and cochlea in a majority of patients. This breakdown of the blood–brain barrier in the enhanced areas is presumably caused by leakage of inflamed microvessels.24,25 The decrease in enhancement with anakinra therapy suggests that these CNS lesions were mediated by interleukin-1β–induced inflammation. Arachnoid adhesions were most likely sequelae of the chronic meningitis that occurs in this disorder26 and may have contributed to the development of increased intracranial pressure, a known complication of chronic meningitis.27 These imaging techniques may be useful in the identification of CNS disease and response to therapy in such patients.
The injection of interleukin-1β into the peripheral circulation causes fever28 and generalized constitutional influenza-like symptoms. This process seems to be dependent on interleukin-6, since fever does not develop in mice that are deficient in interleukin-6,29 despite the fact that interleukin-1β–induced expression of cyclooxygenase-2 and the production of prostaglandin E2 are intact.30 Our patients had interleukin-6 levels in the CNS that were higher than those in the serum by a factor of 7 to 8, suggesting that interleukin-6 is produced locally, as has been described in other CNS diseases.31 Although peripherally produced interleukin-1β may penetrate the CNS, it is possible that interleukin-1β is also produced locally. Interleukin-1β levels in cerebrospinal fluid are undetectable, which is probably secondary to the binding of interleukin-1β to proteins and the soluble interleukin-1 receptor.32 Since low levels of cryopyrin are expressed in the brain,33 an inflammasome could be assembled locally, either in infiltrating inflammatory cells or in CNS cells capable of producing interleukin-1β, such as glial cells.34,35 The striking predilection for cochlear inflammation in neonatal-onset multisystem inflammatory disease could be caused by increased permeability of the blood–brain barrier but also could result from local interleukin-1β production.
Several ophthalmologic symptoms of neonatal-onset multisystem inflammatory disease, including conjunctivitis, uveitis, and corneal infiltrates, rapidly responded to treatment with anakinra. Although no new or progressive loss of peripheral vision was observed during six months of treatment further follow-up is needed to assess the long-term effects of this medication on these and other disease manifestations.
Given the efficacy with which anakinra reduced serum amyloid A levels, study is warranted of whether over the long term, this therapy may prevent systemic amyloidosis, which is reported to occur in as many as 25 percent of patients. Investigation of the use of very early treatment with anakinra before bone lesions develop may help distinguish whether the arthropathy in neonatal-onset multisystem inflammatory disease is driven by interleukin-1β or whether cryopyrin expression in chondrocytes causes impaired apoptosis at the sites of enchondral ossification, as has been suggested.4 In addition, although our study was not powered to detect differences between patients with CIAS1 mutation and patients without such a mutation, the similarity of the underlying disease and therapeutic response to anakinra in the two groups suggests that there may be other disease-associated lesions in the interleukin-1 signaling pathway.
In summary, our study demonstrates that six months of treatment with the interleukin-1β inhibitor anakinra appeared to be safe and highly effective in patients with neonatal-onset multisystem inflammatory disease, including those with neurologic manifestations, who had had incomplete responses to systemic corticosteroids and TNF blockade. Further study is warranted to assess the long-term effects of this treatment in neonatal-onset multisystem inflammatory disease, as well as its role in the treatment of other diseases in which inherited or acquired molecular lesions in interleukin-1 signaling drive inflammation.36
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the NIH.
We are indebted to all the patients with neonatal-onset multisystem inflammatory disease who participated in the study and to their families, whose continuous support and enthusiasm made this research possible.
Footnotes
Dr. Stein reports having received consulting and lectures fees from Amgen and Genentech and research support from Amgen and Abbott; Dr. Moore, lecture fees from Amgen; Dr. Vehe, lecture fees from Amgen and research support from Abbott; and Dr. Cole, consulting fees from Abbott and lecture fees from Amgen. Amgen produces and distributes the medication evaluated in this study. No other potential conflict of interest relevant to this article was reported.
References
- 1.Prieur AM, Griscelli C. Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. J Pediatr. 1981;99:79–83. doi: 10.1016/s0022-3476(81)80961-4. [DOI] [PubMed] [Google Scholar]
- 2.Database of human genes and genetic disorders: OMIM (Online Mendelian Inheritance in Man) Bethesda, Md: National Center for Biotechnology Information; 2006. [Accessed July 14, 2006]. at http://www.ncbi.nlm.nih.gov/entrez/Omim. [Google Scholar]
- 3.Prieur AM, Griscelli C, Lampert F, et al. A chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome: a specific entity analysed in 30 patients. Scand J Rheumatol Suppl. 1987;66:57–68. doi: 10.3109/03009748709102523. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann J, Prieur AM, Quartier P, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–8. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17:586–99. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 9.Manji GA, Wang L, Geddes BJ, et al. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. J Biol Chem. 2002;277:11570–5. doi: 10.1074/jbc.M112208200. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Manji GA, Grenier JM, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–80. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1. 1 is an inducible inflammatory mediator with NF-kappaB suppressive properties. J Immunol. 2003;171:6329–33. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 12.Yu JW, Wu J, Zhang Z, et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappa B, via ASC oligomerization. Cell Death Differ. 2006;13:236–49. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 13.Kanneganti TD, Ozoren N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 14.Grenier JM, Wang L, Manji GA, et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 2002;530:73–8. doi: 10.1016/s0014-5793(02)03416-6. [DOI] [PubMed] [Google Scholar]
- 15.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279:21924–8. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- 16.Lovell DJ, Bowyer SL, Solinger AM. Interleukin-1 blockade by anakinra improves clinical symptoms in patients with neonatal-onset multisystem inflammatory disease. Arthritis Rheum. 2005;52:1283–6. doi: 10.1002/art.20953. [DOI] [PubMed] [Google Scholar]
- 17.Frenkel J, Wulffraat NM, Kuis W. Anakinra in mutation-negative NOMID/CINCA syndrome: comment on the articles by Hawkins et al and Hoffman and Patel. Arthritis Rheum. 2004;50:3738–9. doi: 10.1002/art.20497. [DOI] [PubMed] [Google Scholar]
- 18.Granel B, Serratrice J, Disdier P, Weiller PJ. Dramatic improvement with anakinra in a case of chronic infantile neurological cutaneous and articular (CINCA) syndrome. Rheumatology (Oxford) 2005;44:689–90. doi: 10.1093/rheumatology/keh547. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins J, Gallimore JR, Tennent GA, et al. Rapid automated enzyme immunoassay of serum amyloid A. Clin Chem. 1994;40:1284–90. [PubMed] [Google Scholar]
- 20.Hoffmann SC, Kampen RL, Amur S, et al. Molecular and immunohistochemical characterization of the onset and resolution of human renal allograft ischemia-reperfusion injury. Transplantation. 2002;74:916–23. doi: 10.1097/00007890-200210150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Aganna E, Martinon F, Hawkins PN, et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002;46:2445–52. doi: 10.1002/art.10509. Erratum, Arthritis Rheum 2002;46:3398. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins PN, Lachmann HJ, McDermott MF. Interleukin-1–receptor antagonist in the Muckle–Wells syndrome. N Engl J Med. 2003;348:2583–4. doi: 10.1056/NEJM200306193482523. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–12. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 24.Russell EJ, Geremia GK, Johnson CE, et al. Multiple cerebral metastases: detectability with Gd-DTPA-enhanced MR imaging. Radiology. 1987;165:609–17. doi: 10.1148/radiology.165.3.3317495. [DOI] [PubMed] [Google Scholar]
- 25.Brekenfeld C, Foert E, Hundt W, Kenn W, Lodeann KP, Gehl HB. Enhancement of cerebral diseases: how much contrast agent is enough? Comparison of 0.1, 0.2, and 0.3 mmol/kg gadoteridol at 0.2 T with 0.1 mmol/kg gadoteridol at 1. 5 T. Invest Radiol. 2001;36:266–75. doi: 10.1097/00004424-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Frosch MP, Anthony DC, de Girolami U. The central nervous system. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran pathologic basis of disease. 7. Philadelphia: Elsevier Saunders; 2005. pp. 1347–420. [Google Scholar]
- 27.Gripshover NM, Ellner JJ. Chronic meningitis. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 5. Philadelphia: Churchill Livingstone; 2000. pp. 998–1000. [Google Scholar]
- 28.Luheshi GN. Cytokines and fever: mechanisms and sites of action. Ann N Y Acad Sci. 1998;856:83–9. doi: 10.1111/j.1749-6632.1998.tb08316.x. [DOI] [PubMed] [Google Scholar]
- 29.Kagiwada K, Chida D, Sakatani T, et al. Interleukin (IL)-6, but not IL-1, induction in the brain downstream of cyclooxygenase-2 is essential for the induction of febrile response against peripheral IL-1alpha. Endocrinology. 2004;145:5044–8. doi: 10.1210/en.2004-0054. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Ballou LR, Morham SG, Blatteis CM. Cyclooxygenase-2 mediates the febrile response of mice to interleukin-1beta. Brain Res. 2001;910:163–73. doi: 10.1016/s0006-8993(01)02707-x. [DOI] [PubMed] [Google Scholar]
- 31.Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992;263:C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–99. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JP, Mueller JL, Rosengren S, et al. Structural, expression, and evolutionary analysis of mouse CIAS1. Gene. 2004;338:25–34. doi: 10.1016/j.gene.2004.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–4. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- 35.Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–71. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–9. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.