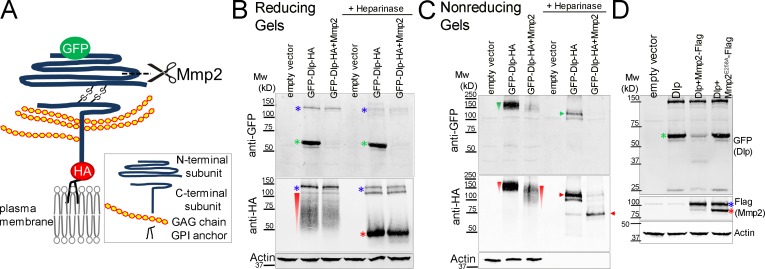
Figure 5.
Mmp2 cleaves the N-terminal subunit of Dlp in cultured cells. All Western blots were performed on transiently transfected S2R+ cell lysates. (A) Diagram of a Dlp construct. Mature Dlp is processed by furin-like convertase into N- and C-terminal subunits that are linked by two disulfide bonds. GFP labels the N terminus and HA labels the C terminus. The cleavage by Mmp2 as deduced from experiments in B and C is shown. (B) On reducing gels, the N-terminal subunit ran at ∼60 kD (green asterisks); the GAG modifications on the C-terminal subunit caused it to run as a smear (red wedge), resolved by heparinase treatment to a ∼50 kD band (red asterisk). Mmp2 overexpression eliminated detection of the GFP-labeled N-terminal subunit (lanes 3 and 6). The high molecular weight bands (blue asterisk) probably represent unprocessed Dlp precursor polypeptide (see also Fig. S5 C). (C) On nonreducing gels, the disulfide-bonded Dlp protein ran as a smear at ∼150 kD (green and red wedges), resolved by heparinase into doublets (green and red arrow heads) between 100 kD and 150 kD. Mmp2 overexpression reduced the sizes of the Dlp smear (lane 3, red wedge) and band (lane 6, red arrowhead) recognized by HA. (D) Dlp cleavage required Mmp2 catalytic activity, as Mmp2E258A did not decrease the level of GFP-labeled N-terminal subunit (compare lanes 3 and 4). Flag-tagged Mmp2 ran as doublets (the blue asterisk represents the likely zymogen and the red asterisk represents the likely activated enzyme without the pro-domain). Actin, loading control.