Fig. 4.
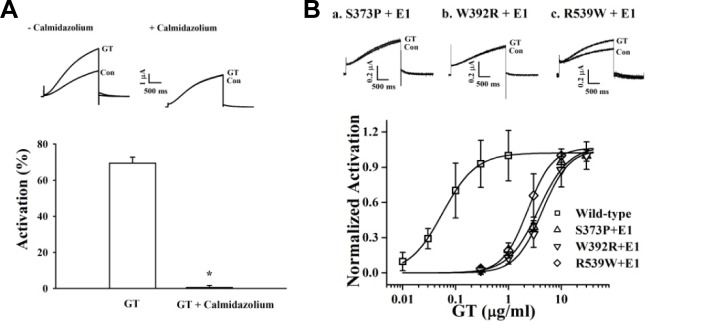
Involvement of CaM in gintonin-mediated IKs channel activation. (A) Oocytes expressing IKs channels were incubated in the absence or presence of calmidazolium (1.5 μM) for 10 min. Insets, the representative gintonin-mediated mediated peak outward current amplitude at +30 mV from a holding potential of −80 mV was measured in the absence or presence of calmidazolium. Summary histograms show peak outward IKs channel currents recorded in the absence or presence of calmidazolium (mean ± S.E.M; n = 13–14 oocytes each; *P < 0.001, compared to gintonin alone). (B) Oocytes expressing IKs channels mutated at the Ca2+/CaM-binding sites (S373P, W392R, or R539W) were treated with gintonin for 60 s. Mutation of Ca2+/CaM-binding sites resulted in a rightward shift of the gintonin concentration-response curve (mean ± S.E.M; n = 10–12 oocytes each). Insets, the representative peak outward current amplitude at +30 mV from a holding potential of −80 mV was measured in the presence of gintonin. Gintonin-mediated peak outward IKs channel currents recorded in oocytes expressing mutant channels were significantly attenuated (mean ± S.E.M; n = 10–12 oocytes each; *P < 0.001, compared to the wild type).
