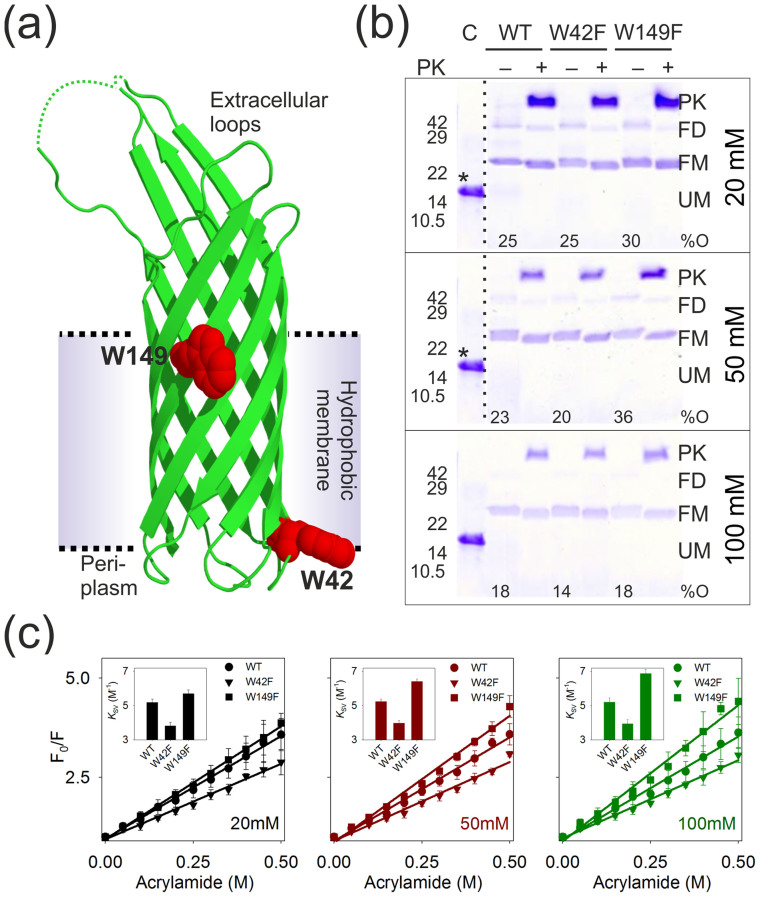
Figure 1. Response of Trp residues of Ail to DPR and its influence on Ail refolding.
(a) Ribbon diagram of Ail obtained from the crystal structure (PDBID: 3QRA25) highlighting the two Trp residues as red spheres. The putative transmembrane segment is also indicated. Segments of two extracellular loops, absent in the structure, are denoted as dotted lines. (b) SDS-PAGE gel mobility shift assay of unboiled Ail-WT, W42F and W149F samples refolded in 20 mM (top), 50 mM (middle) and 100 mM (bottom) LDAO. Refolded samples also subjected to pulse proteolysis using proteinase K (PK) are indicated as (+) and undigested samples as (-). Bands corresponding to the folded dimer (FD) and higher order oligomers are most prominent in the 20 mM LDAO sample. Refolded monomer (FM), migrating at ~24 kDa, and the unfolded monomer (UM) migrating at ~17.0 kDa, are also marked. Unfolded Ail in 8.0 M urea served as the control (C). The percentage of observed Ail oligomers (%O) in each condition was quantified by densitometry42, and is provided below the respective sample lanes. Band positions of molecular weight standards are indicated beside each gel. The unfolded control from the bottom gel has been shown in the top and middle gels, and is marked (*). Dotted lines separate different sections of gels that have been presented together. Note that the various DPRs underwent different dilutions before SDS-PAGE (to minimize interference from LDAO on band migration), and therefore protein concentrations across the three gels are not similar. (c) Plot of Trp fluorescence quenching of Ail-WT (circles), W42F (inverted triangles) and W149F (squares) with increasing acrylamide concentrations, fitted to a linear equation (fits are shown as lines), to obtain the Stern-Volmer constant (KSV) (shown as inset). Shown here are data obtained in 20 mM (black), 50 mM (red) and 100 mM (green) LDAO. Note that the W149F construct displays an increase in KSV upon addition of LDAO, whereas corresponding values for the WT and W42F proteins are largely DPR insensitive (also see Supplementary Fig. 1). KSV values for unfolded Ail (in 8.0 M GdnHCl; all three constructs) was ~7.4 M−1 and aggregated Ail was ~3.1 M−1.