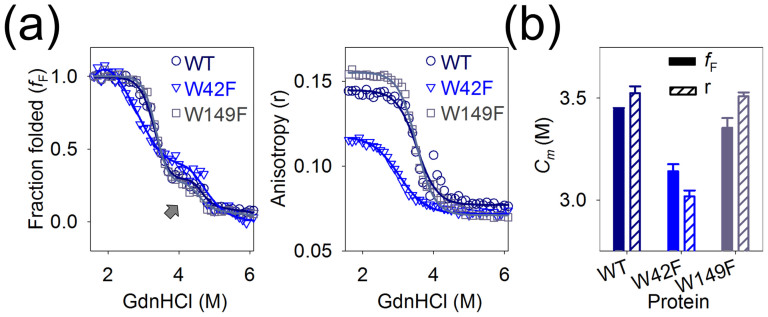
Figure 3. A refolding intermediate of Ail is observed in high DPR.
(a) Comparison of the folded fraction (fF) and anisotropy values of Ail-WT ( , purple) and the Trp mutants W42F (
, purple) and the Trp mutants W42F ( , blue) and W149F (
, blue) and W149F ( , grey) refolded in 200 mM LDAO (DPR 7000:1) indicates the presence of a stable intermediate species in the fF (indicated by an arrow), which is absent in the anisotropy (r) data. Fits are indicated by solid lines that are color-coded to match the corresponding symbols. (b) Plot of the Cm values calculated from the fF (solid fill) and anisotropy (r) (diagonal stripes) data using linear extrapolation. Color codes are matched to Fig. 3a. For the fF data, Cm reflects the mid-point of the transition of the ‘adsorbed' protein intermediate to the fully refolded β-barrel form. The lowest Cm is seen in the case of W42F, suggesting destabilization of this protein in very high DPR. Also see Supplementary Figs. 2–4.
, grey) refolded in 200 mM LDAO (DPR 7000:1) indicates the presence of a stable intermediate species in the fF (indicated by an arrow), which is absent in the anisotropy (r) data. Fits are indicated by solid lines that are color-coded to match the corresponding symbols. (b) Plot of the Cm values calculated from the fF (solid fill) and anisotropy (r) (diagonal stripes) data using linear extrapolation. Color codes are matched to Fig. 3a. For the fF data, Cm reflects the mid-point of the transition of the ‘adsorbed' protein intermediate to the fully refolded β-barrel form. The lowest Cm is seen in the case of W42F, suggesting destabilization of this protein in very high DPR. Also see Supplementary Figs. 2–4.