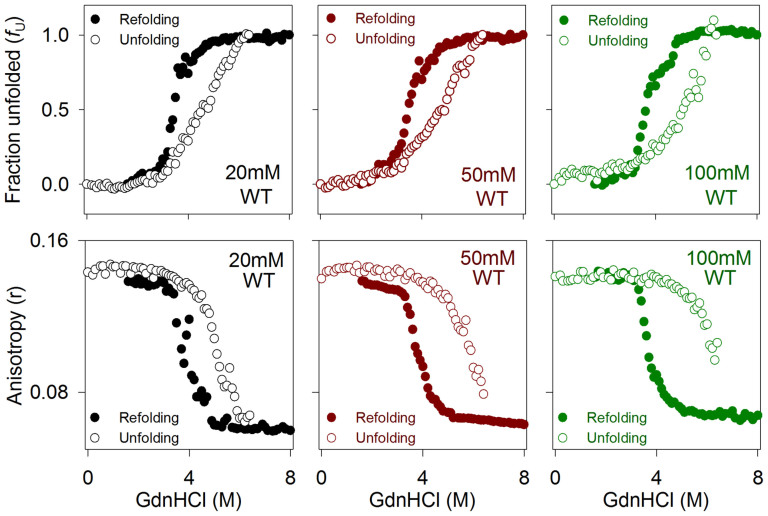
Figure 5. Hysteresis in Ail arises from variations in barrel-micelle interaction efficacy.
(Top) Comparison of the GdnHCl-mediated refolding (filled circles) and unfolding (open circles) curves, shown here for Ail-WT. Data were obtained from unfolded fractions (fU) derived from Trp fluorescence, in 20 mM (black), 50 mM (red) and 100 mM (green) LDAO. The mean values from independent experiments are provided here, without error bars. Notice the cooperativity of the refolding reaction, and the comparatively low cooperativity of the unfolding reaction. (Bottom) Representative Trp anisotropy values obtained for the GdnHCl-mediated refolding (filled circles) and unfolding (open circles) of WT Ail. Note that while the fU data for the unfolding experiment tends to 1.0 in 100 mM LDAO (top panel), the corresponding anisotropy values do not reach ~0.06 (for a fully unfolded protein), suggesting that unfolded Ail probably binds substantially to LDAO in the vicinity of the aromatics. A similar color scheme is retained in all datasets. Also see Supplementary Fig. 13.