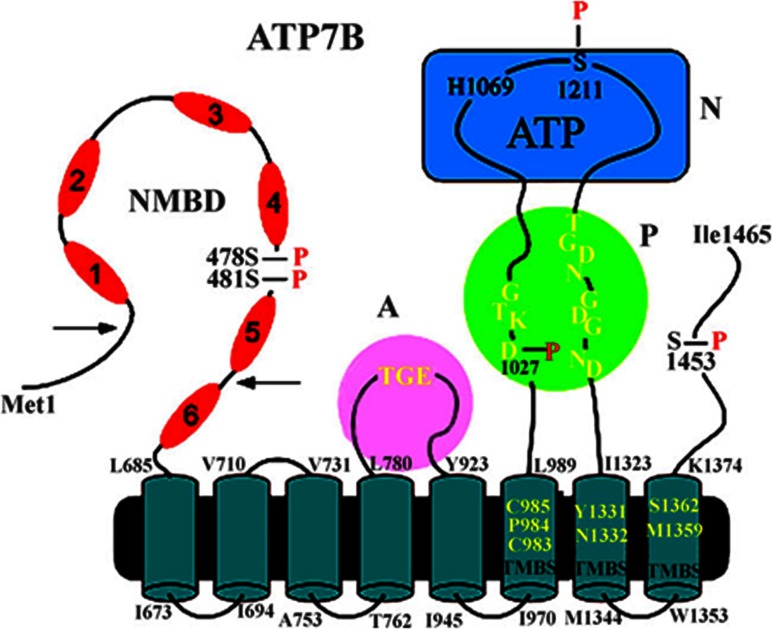
Figure 4. 2D folding model of the (mammalian) ATP7B sequence.
The diagram shows eight transmembrane segments including a copper-binding site (TMBS). Yellow residues in the TMBS are probably involved in copper binding. The extramembranous region comprises a nucleotide-binding domain (N) with the His1069 residue whose mutation is frequently found in Wilson's disease; the P domain, with several residues (in yellow) conserved in P-type ATPases, where Asp1027 undergoes phosphorylation to form the catalytic phosphoenzyme intermediate (EP); the A domain with the TGE (yellow) conserved sequence involved in catalytic assistance of EP hydrolytic cleavage; the NMBD with six copper-binding sites; and a C-terminal chain. As suggested in [46], a kinked amphipathic segment of the second helix may overlap the cytosolic membrane surface. Serine residues undergoing kinase-assisted phosphorylation (Ser478, Ser481, Ser1121 and Ser1453) reside within flexible loops of the protein. This Figure was adapted from J. Biol. Chem. [55] Pilankatta, R., Lewis, D., Adams, C.M. and Inesi, G. C. High yield heterologous expression of wild-type and mutant Cu+-ATPase (ATP7B, Wilson disease protein) for functional characterization of catalytic activity and serine residues undergoing copper-dependent phosphorylation. Journal of Biological Chemistry. 2009; 284, 21307–21316. © the American Society for Biochemistry and Molecular Biology and J. Biol. Chem [60] Pilankatta, R., Lewis, D. and Inesi G. Involvement of protein kinase D in expression and trafficking of ATP7B (copper ATPase). Journal of Biological Chemistry. 2011; 286, 7389–7396