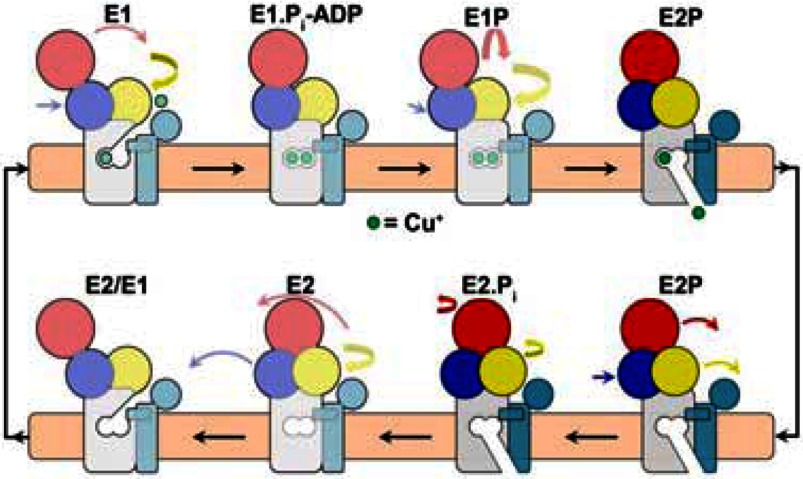
Scheme 3. Reaction scheme proposed for the L. pneumophila CopA, where the headpiece domains are shown in yellow (A), red (N), blue (P) and light blue (NMBD), and the coloured arrows indicate their movements.
Note that the transmembrane domains undergo no major movements in the transition between E2-P and E2.Pi. However, as explained above, conformational transitions occur upon Cu+ binding to the NMBD and TNBS, as well as upon utilization of ATP. Most importantly, the (Cu•) E1 P to (Cu•) E2-P conformational transition is related to utilization of phosphorylation potential, resulting in vectorial displacement of bound Cu+. Reprinted by permission from Macmillan Publishers Ltd: Nature Structural and Molecular Biology [35] Andersson, M., Mattle, D., Sitsel, O., Klymchuk, T., Nielsen, A. M., Møller, L. B., White, S. H., Nissen, P. and Gourdon, P. (2014) Copper-transporting P-type ATPases use a unique ion-release pathway. Nat. Struct. Mol. Biol. 21, 43–48, copyright (2014)