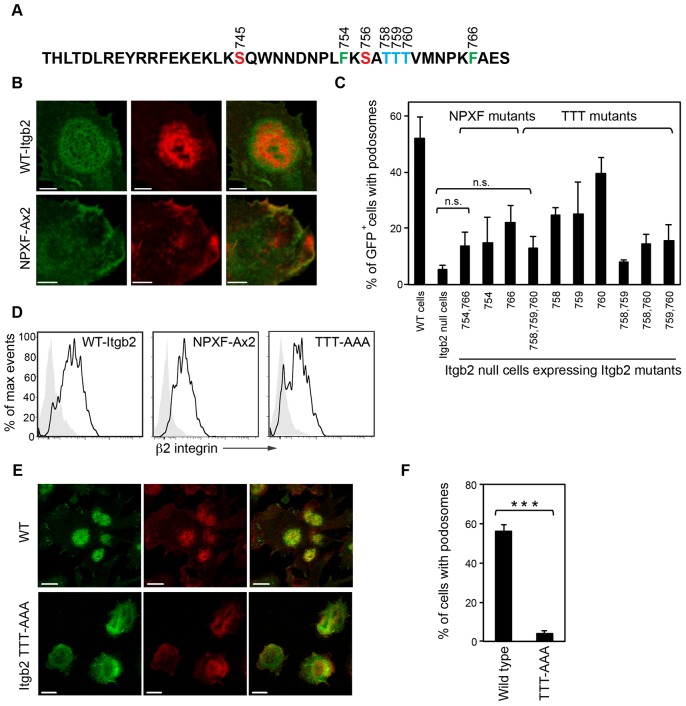
Fig. 5.
Mutation of key residues in the cytoplasmic tail of Itgb2 and their effect on podosome formation. (A) Amino acid sequence of the cytoplasmic tail of the mouse β2 integrin with residues of interest highlighted; Ser745, Ser756 (red), Thr758, Thr 759, Thr 760 (TTT; blue), Phe754 and Phe766 (green). Amino acid positions are based on the human β2 integrin. (B) Itgb2-null cells were reconstituted with either wild type Itgb2-EGFP (WT-Itgb2) or NPXF-Ax2-Itgb2-EGFP (NPXF-Ax2) by retroviral infection (green). After fixation, the cells were stained for F-actin (red; Alexa-Fluor-555). The Itgb2-NPXF-A mutant was unable to rescue podosome formation even though it localized to the plasma membrane. (C) Percentage of podosomes in control WT cells or Itgb2-null cells reconstituted with GFP alone or with Itgb2 constructs containing the following cytoplasmic tail mutations as indicated. NPxF-Ax2: F754A, F766A. TTT-AAA: T758A, T759A, T760A, and double and triple combinations thereof. Data are from 100–150 GFP-positive cells per sample. Podosomes were not significantly reconstituted in Itgb2-null cells expressing the NPXF-Ax2 or TTT-AAA mutants compared to GFP alone (paired t-tests). (D) Cell surface expression of the WT-Itgb2 and NPxF-Ax2 and TTT-AAA mutants in the infected cells was confirmed by flow cytometry using an anti-β2 integrin antibody. (E) BMDCs cultured from Itgb2WT or Itgb2 TTT-AAA β2 cytoplasmic tail knock-in mice were stained for β2 integrin (green; FITC) and F-actin (red; Alexa-Fluor-555). Scale bars: 5 µm (B) and 10 µm (E). (F) Percentage of cells with podosomes indicates a loss of the podosome phenotype in the TTT-AAA knock-in DCs (***P<0.001, unpaired t-test). Images were acquired using a Zeiss LSM700, as in Fig. 1.