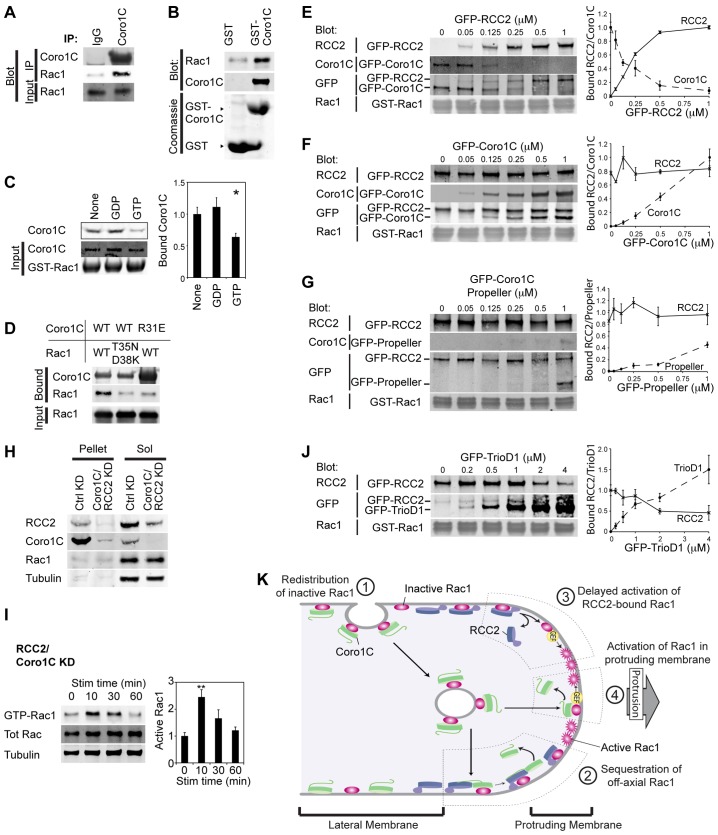
Fig. 6.
RCC2 and Coro1C compete for an overlapping binding site on Rac1. (A) Endogenous Coro1C and Rac1 co-immunoprecipitated (IP) from fibroblasts, n = 4. (B) Recombinant GDP-loaded Rac1 bound directly to GST–Coro1C, n = 7. (C) GFP–Coro1C from 293T lysates bound poorly to GTPγS-loaded, compared to GDP-loaded or nucleotide-free GST-Rac1, n = 6. (D) Interaction between GFP–Coro1C and recombinant GDP-loaded Rac1 was blocked by mutation of an interaction site of either molecule, n = 4. WT, wild-type. (E–G,J) GST–GDP-Rac1 beads loaded with 0.25 µM GFP–Coro1C or GFP–RCC2 in the presence of increasing concentrations of GFP–RCC2, GFP–Coro1C, GFP–Coro1C propeller or GFP–TrioD1 competitor, n = 4. Graphs depict average relative quantitation of bound protein in GFP blots. (H) Rac1 remained in the detergent-soluble (Sol) fraction upon knockdown (KD) of both Coro1C and RCC2, n = 11. (I) Syndecan-4-stimulated (Stim) Rac1 activation in RCC2- and Coro1C-double-knockdown MEFs, n = 6. *P<0.05, **P<0.005 (ANOVA). Results are mean±s.e.m. (K) Schematic of the roles of Coro1C and RCC2 in Rac1 retrafficking and sequestration.