Fig. 1.
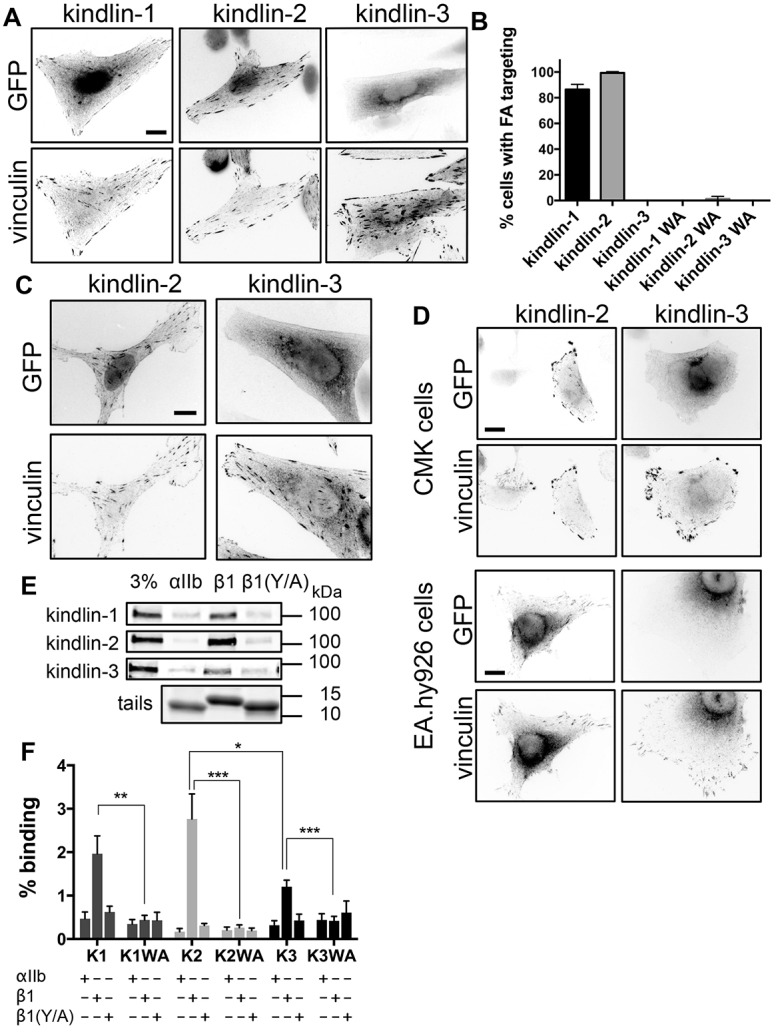
Kindlin isoforms have different subcellular localization and integrin binding. (A) CHO cells overexpressing GFP-tagged kindlins were plated on fibronectin, fixed 24 h later and stained for endogenous vinculin. (B) Percentage of transfected cells (±95% confidence interval) in which the GFP-tagged kindlins and mutants (kindlin-1-W612A, kindlin-2-W615A and kindlin-3-W597A, indicated by WA) target to FAs. n = 160–280 cells from three independent experiments. (C) αIIbβ3-integrin-expressing CHO cells overexpressing GFP-tagged kindlins were plated on fibrinogen, fixed and stained for endogenous vinculin. (D) CMK or EA.hy926 cells overexpressing GFP-tagged kindlins were plated on fibronectin and stained for endogenous vinculin. (E) Pulldown of GFP-tagged kindlins from CHO cell lysates with purified recombinant αIIb, β1 or β1Y795A (β1Y/A) integrin tails. Tail loading was assessed by Coomassie Blue staining. The 3% lane indicates 3% of input lysate. (F) GFP–kindlin-1 (K1), GFP–kindlin-1-W612A (K1WA), GFP–kindlin-2 (K2), GFP–kindlin-2-W615A (K2WA), GFP–kindlin-3 (K3) and GFP–kindlin-3-W597A (K3WA) binding to purified recombinant αIIb, β1 and β1Y/A tails was quantified and expressed as a percentage of input (mean±s.e.m.; n = 4). *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). Scale bars: 10 µm.
