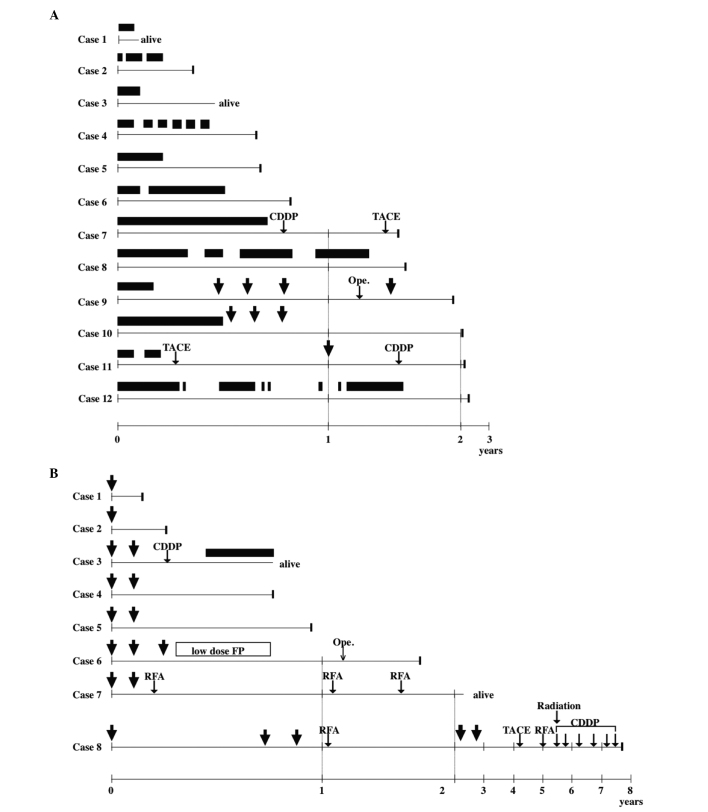
Figure 1.
Clinical course of (A) the sorafenib and (B) hepatic arterial infusion chemotherapy (HAIC) groups. The best clinical responses were complete response in 1 patient (case 8) in the HAIC group, partial response in 2 patients (cases 6 and 7) in the HAIC group, stable disease in 10 patients (cases 1, 6, 9, 10, 11 and 12 in the sorafenib group and cases 2, 3, 4 and 5 in the HAIC group) and progressive disease in 7 patients (cases 2, 3, 4, 5, 7 and 8 in the sorafenib group and case 1 in the HAIC group). Although patients 1 and 3 in the sorafenib group and patients 3 and 7 in the HAIC group remained alive, other patients succumbed to the disease at the indicated time points. Closed bars, sorafenib administration. Arrows, HAIC. CDDP, cisplatin infusion; TACE, transcatheter arterial chemoembolization; Ope., operation; low-dose FP, continuous 5-fluorouracil and low-dose cisplatin infusion; RFA, radiofrequency ablation.