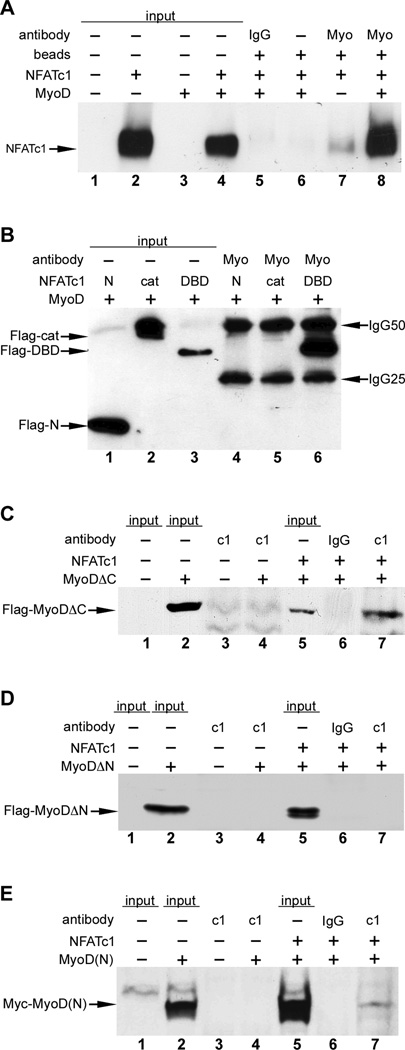
Figure 3. NFATc1 physically interacts with the N-terminus of MyoD.
C3H10T1/2 cells were transfected with the indicated plasmids, and lysates were analyzed by immunoprecipitation (IP)-western blot. Sample inputs, IgG antibody controls, and beads only controls are indicated. MyoD physically interacted with full length NFATc1 (panel A, lane 8). IP, anti-MyoD; western, anti-NFATc1. MyoD interacts with the DNA binding domain (DBD) of NFATc1 (panel B, lane 6). Expression plasmids for full length MyoD and Flag-tagged truncation fragments of NFATc1, encoding the N-terminus (N), catalytic domain (cat) or DBD were cotransfected. IP, anti-MyoD; western, anti-Flag. (C–E) Expression plasmids for full length NFATc1 and Flag-tagged MyoD lacking the C-terminus (C), Flag-tagged MyoD lacking the N-terminus (D) or a Myc-tagged N-terminal fragment of MyoD (E) were cotransfected. IP, anti-NFATc1; western, anti-Flag (panels C and D), anti-Myc (panel E). MyoDΔC was efficiently co25 immunoprecipitated by anti-NFATc1 (panel C, lane 7). The N-terminus of MyoD alone [MyoD(N)] was co-immunoprecipitated with NFATc1 (panel E, lane 7). MyoDΔN was not co-immunoprecipitated by anti-NFATc1 (panel D, lane 7).