Abstract
Maternal administration of betamethasone to enhance fetal lung maturation for women who threaten preterm labor is common clinical practice. However, recommendations regarding the choice of betamethasone formulations for perinatal use are vague. The disposition of betamethasone from two commonly used antenatal formulations is poorly understood. We therefore designed a study to capture the true pharmacokinetic profiles of betamethasone from these fast acting and dual-release formulations. Betamethasone in sheep plasma was measured by a newly designed, highly sensitive liquid chromatography/tandem mass spectrometry assay after intramuscular injection (n = 4) of 0.25 mg/kg betamethasone phosphate and 0.5 mg/kg betamethasone phosphate/acetate formulations. Compartmental modeling was performed using the ADAPT II program. Betamethasone pharmacokinetics could be captured for 24 h for the phosphate and for 5 days for the phosphate/acetate formulations. The phosphate formulation profile had the appearance of a traditional Bateman function with a terminal half-life of 4 h, whereas the phosphate/acetate formulation produced a biexponential decline with a terminal half-life of 14 h. The latter is much longer than is commonly reported and has been missed in the literature due to assay limitations. Extrapolations to humans indicate that although both formulations might have similar therapeutic indices, the dual formulation might be associated with a lower safety profile. In light of this newly identified long terminal half-life for the betamethasone dual formulation, dosing practices for betamethasone in pregnancy need to be reassessed.
Preterm birth occurs in about 10% of pregnancies, and complications associated with prematurity, especially respiratory distress syndrome, are the leading cause of mortality in prematurely born infants (NIH Consensus Panel, 1995). Betamethasone is administered maternally to enhance fetal lung maturation in women who threaten preterm labor during 24 to 34 weeks gestation. The National Institutes of Health recommends administration of two maternal intramuscular injections of 12-mg betamethasone 24 h apart for this condition. Although the doses of betamethasone are stated, the exact formulation recommendation is not clear. Betamethasone is available as a fast releasing phosphate ester prodrug formulation and as a dual acting suspension formulation containing phosphate and acetate ester prodrugs. Both formulations have been tested in clinical trials and have been shown to be efficacious in producing precocious fetal lung maturation (Liggins and Howie, 1972; Gamsu et al., 1989). However, there is controversy regarding the betamethasone-releasing properties of the acetate prodrug, and a recent meta-analysis suggests that this prodrug is probably of little therapeutic benefit for antenatal use (Jobe and Soll, 2004). Although the release properties of the acetate prodrug have been questioned, long-duration studies looking at the release pattern of betamethasone from this prodrug do not exist. Furthermore, traditional chromatographic assays for betamethasone in animal studies suffer from sensitivity and sample stability issues that were raised in a recent meta-analysis (Samtani et al., 2004a). These problems remain unresolved, and we therefore designed a study to capture the true pharmacokinetic profiles of betamethasone from the two formulations. The study design and rationale were as follows. 1) The most commonly utilized animal model for fetal maturation studies is the pregnant sheep (Dunlop et al., 1997) and, hence, the pharmacokinetic investigation was conducted in ewes. 2) The formulations were compared in nonpregnant animals. Our meta-analysis (Samtani et al., 2004a) has pointed out that complete pharmacokinetic characterization of drugs in pregnancy requires complicated study designs involving maternal/fetal dosing and sampling. Corticosteroid studies in sheep pregnancy are complicated by the use of tocolytics to prevent premature labor, require technical experience with ultrasound-guided fetal injections, and require a 100- to 120-day wait period to reach the appropriate gestational age for corticosteroid studies. We aspired to capture betamethasone release from the acetate prodrug, and this objective can be easily accomplished by studying pharmacokinetics in the uncomplicated nonpregnant state. 3) The dual acting formulation was evaluated as a single intramuscular injection of 0.5 mg/kg. This dose is higher than that recommended by the National Institutes of Health in humans (0.17 mg/kg), but has been found to be the dosage in sheep that consistently produces preterm fetal lung maturation (Moss et al., 2001). This is also the highest dose level that can be investigated because it is associated with an injection size of 4 to 6 ml, which is the maximum recommended intramuscular administration volume (Rodger and King, 2000). The use of a high dose is necessary to capture the low-level betamethasone release that could occur from the acetate prodrug. The dose of the fast acting phosphate formulation was chosen to be 0.25 mg/kg. The 0.5 mg/kg injection of the dual formulation contains 0.25 mg/kg phosphate prodrug. Thus, betamethasone release by the acetate prodrug can be easily obtained by simple subtraction of the two profiles obtained from the two dose groups. 4) We designed a liquid chromatography/tandem mass spectrometry (LC/MS/MS) assay for measuring betamethasone that is capable of measuring low drug concentrations. This would help with the aim of measuring slow release of betamethasone from the acetate prodrug. 5) To overcome the problem of sample stability, plasma obtained from both formulation groups was stabilized using a 100 mM concentration of a phosphatase inhibitor, sodium arsenate (Samtani et al., 2004b). In addition, samples from the dual prodrug group were stabilized with 86 mM potassium fluoride, which inhibits plasma esterases (Petersen et al., 1980).
Materials and Methods
Materials
Sodium arsenate heptahydrate, potassium fluoride, formic acid, and prednisolone internal standard were purchased from Sigma-Aldrich (St. Louis, MO). The source for betamethasone was Steraloids (Newport, RI). High-performance liquid chromatography grade water and acetonitrile were obtained from Honeywell Burdick & Jackson (Muskegon, MI). Methanol was obtained from EMD Chemicals Inc. (Gibbstown, NJ). Phosphoric acid was obtained from J. T. Baker (Phillipsburg, NJ), and ammonium formate was supplied by Fluka (Buchs, Switzerland). Clinically used formulations for betamethasone phosphate (Celestan Solubile) and betamethasone phosphate/acetate (Celestan Depot) were obtained from Essex Pharma (Munich, Germany).
Betamethasone Pharmacokinetic Study
Experimental procedures were approved by the Animal Care and Use Committee of the University of Wyoming, Laramie, WY. Eight Western Range ewes were weighed and brought into the animal facilities 1 day before the experiment. The animals were housed in outside pens and had ad libitum access to hay, hay cubes, and water. On the day of the experiment, ewes were randomly assigned to two groups of four animals and were treated as follows. Group 1 received 0.25 mg kg−1 betamethasone phosphate, and the mean body weight of the animals in this group was 70 ± 6.4 kg (mean ± S.D.). Group 2 received 0.5 mg kg−1 betamethasone phosphate/acetate mixture, and the average body weight of the animals was 68 ± 9.4 kg. The injections were administered intramuscularly into the gluteal muscle at 8:30 AM to minimize the impact of circadian cortisol rhythms on the results. In both groups, blood samples were taken directly from the jugular vein at 15, 30, 45, 60, and 90 min and at 3, 5, 8, 12, and 24 h after each injection. In the dual formulation group, additional blood samples were taken 2, 3, 4, 5, 6, 7, 8, 10, 12, and 14 days after the injection. Blood samples were collected in prechilled EDTA tubes and immediately put on ice after 50 µl of 2 M sodium arsenate solution/ml of blood was added for stabilization. After centrifugation, plasma was siphoned into plastic tubes and stored at −20°C. In the dual formulation group, 10 µl of 50% (w/v) potassium fluoride solution/ml of plasma was added to all the samples before freezing. Potassium fluoride was added to plasma and not to blood because addition of this stabilizer to blood causes lysis of red blood cells. Plasma samples were shipped on dry ice by overnight courier to Buffalo (State University of New York), where they were stored at −20°C until assayed by our LC/MS/MS method.
Additional Data Source
The maternal betamethasone profile after maternal intramuscular administration of 0.5 mg/kg phosphate/acetate formulation in pregnant sheep was obtained from a recently conducted meta-analysis (Samtani et al., 2004a).
Sample Preparation
Sample processing was carried out in laboratory plasticware to prevent the known adsorption of steroids to glassware (Makin et al., 1995). Nonspecific adsorption can affect analyte sensitivity and recovery in the low-level analysis described in this work, and the hydrophobicity of the polypropylene plastic surface helps reduce sample adsorption problems (Tsutsumi et al., 2003). Sample preparation involved adding 0.5 ml of 4% phosphoric acid to 0.5 ml of plasma sample in polypropylene tubes. This makes the samples less viscous and can free up the protein-bound drug in plasma (Ding and Neue, 1999). As internal standard, 50 µl of a methanolic stock (1 µg/ml) of prednisolone was added. After thorough mixing, samples were centrifuged at 8000g for 20 min and then subjected to solid-phase extraction using Oasis HLB 1 cc, 30 mg cartridges (Waters, Milford, MA). The extraction was carried out on a Vac Elut SPS 24 solid-phase extraction manifold (Varian, Inc., Palo Alto, CA). The samples were extracted using the generic Oasis HLB procedure recommended by the manufacturer for 1 cc cartridges. Briefly, the SPE cartridge was preconditioned with 1 ml of methanol, followed by 1 ml of water. One milliliter of the processed sample was pulled through the cartridge, the cartridge was washed with 1 ml of 5% methanol in water, and elution was performed with 1 ml of methanol. The methanolic eluant was dried at 50°C under a gentle nitrogen stream, and the dried residue was reconstituted with 50% 10 mM ammonium formate and 0.1% formic acid/50% acetonitrile. The reconstituted samples were transferred into 0.5-ml polypropylene tubes, centrifuged at 16,000g for 10 min at 4°C, and finally injected into the LC/MS/MS apparatus.
Plasma-based standards and quality control (QC) standards were prepared from blank sheep plasma. Betamethasone methanolic stock solutions were added to polypropylene tubes and dried under nitrogen. Appropriate volumes of plasma were added, the tubes were vortexed, and QC/standards were aliquoted at >0.5 ml into 1.5-ml polypropylene tubes and stored at −20°C. The assay covers a concentration range of 0.1 to 100 ng/ml for betamethasone. Samples expected to have concentration greater than 100 ng/ml were diluted with an appropriate volume of blank plasma. Standards were run on a daily basis, and samples and QC were quantified using a quadratic regression curve of analyte to internal standard area ratio versus concentration with a weighting factor of 1/Y2. The assay produced standard curves with r2 ≥ 0.99, had an inter- and intra-assay accuracy and precision of ≤14%, and offered almost complete extraction recovery for betamethasone.
LC/MS/MS Analysis
Analysis was performed on a system equipped with an Agilent Technologies (Palo Alto, CA) model 1100 autosampler, a dual pump, and an Applied Biosystems MDS/Sciex (Foster City, CA) API 3000 mass spectrometer using a turbo-ion spray source. The system control and data analysis were executed using the Analyst software (Applied Biosystems, Version 1.4). Chromatography was performed on a C8 Hydrobond AQ column (particle size 3 µm, 2.1 × 150 mm; MAC-MOD Analytical, Inc., Chadds Ford, PA) equipped with a ColumnSaver precolumn filter (MAC-MOD Analytical, Inc). The mobile phase flow rate was 0.2 ml/min with eluant A consisting of 10 mM ammonium formate and 0.1% formic acid, and eluant B consisting of acetonitrile. The mobile phase flow design was as follows: 0 to 4.5 min, 40% A/60% B; 4.6 to 6.0 min, 10% A/90% B to allow system cleanup; followed by a 4-min equilibration step at 40% A/60% B. The mass spectrometer was operated in the positive ionization mode. The optimal ion pairs, declustering potential, collision energy, collision exit potential, focusing potential, and excitation potential for betamethasone and prednisolone were found to be 393.3/373.3, 35 V, 15 V, 23 V, 300 V, and 10 V, and 361.3/343.5, 25 V, 15 V, 20 V, 300 V, and 10 V, respectively. High purity nitrogen was used as the curtain and collision gas. The source temperature was set at 350°C.
Pharmacokinetic Analysis
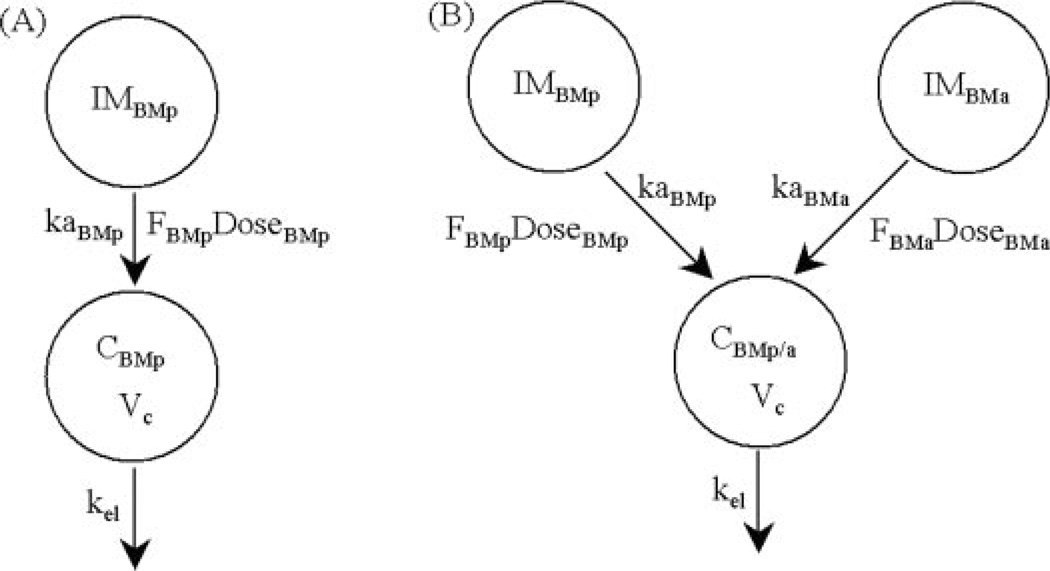
Mean betamethasone concentration profiles as a function of time (t) after administration of the two formulations were fitted simultaneously using the models shown in Fig. 1. The differential equations and their initial conditions used in the fitting procedure were as follows:
| (1) |
| (2) |
where CBMp and CBMp/a refer to concentrations of betamethasone after administration of phosphate and phosphate/acetate formulations, kaBMp and kaBMa are hybrid first-order rate constants representing activation and absorption of betamethasone after intramuscular administration of phosphate and acetate prodrugs, DoseBMp and DoseBMa are the doses of phosphate and acetate prodrugs in terms of betamethasone equivalents, and Vc/FBMp and Vc/FBMa are the apparent volumes of distribution for betamethasone after administration of the betamethasone phosphate and acetate. Two different Vc/F terms are necessary because the two prodrugs may be activated to different extents in vivo. Finally, kel is the elimination rate constant for betamethasone. Absorption and elimination half-lives were calculated as secondary parameters using the formula 0.693/k. Relative bioavailability (FBMa/FBMp) of the acetate versus the phosphate prodrug was obtained from the ratio of the two apparent volumes of distribution. The pharmacokinetic modeling was performed using the maximum likelihood estimator within the ADAPT II computer program (D’Argenio and Schumitzky, 1997). The variance model was:
| (3) |
where Coefficient and Power are variance parameters that were fitted, and Y(t) represents the model output function. The goodness of fit was assessed using correlation coefficients, examination of residuals, and visual inspection.
Fig. 1.
Pharmacokinetic models for characterizing the time course of betamethasone concentrations after intramuscular dosing of 0.25 mg/kg betamethasone phosphate (A) and intramuscular dosing of 0.5 mg/kg betamethasone phosphate/acetate dual formulation (B).
Extrapolation of Pharmacokinetic Profiles to Humans
It will be shown later that the pharmacokinetics of betamethasone is not affected by pregnancy. Thus, extrapolation of results from this study to the pharmacokinetic behavior of the two formulations in human pregnancy can be attempted. Allometric scaling is unnecessary because of the similarity in body weight of the ewes used in this study with that of humans. To project human profiles, drug concentrations need to be scaled to the clinical dose and corrections made for plasma protein binding to obtain free drug concentrations that drive corticosteroid effects. An estimate of possible fetal drug exposure can be obtained by recognizing that the placental barrier creates a fetal to maternal betamethasone concentration gradient in humans of approximately one-third (Samtani et al., 2004a). The correction factors needed to make human extrapolations are provided in Table 1. The calculations were performed in an Excel spreadsheet (Microsoft Excel software; Microsoft, Redmond, WA) by multiplying the combined correction factors with the appropriate predicted concentrations from the model fittings. To understand the relationship between projected concentrations and clinical outcome, efficacy and toxicity indices were calculated. Adrenal suppression was considered as a biomarker for assessing corticosteroid adverse effects. Prednisolone IC50,Free (free drug concentration causing 50% inhibition of cortisol synthesis rate) for adrenal suppression is known to be 1 ng/ml (Wald et al., 1992). Betamethasone, based on relative receptor affinity values, has a 3.6-fold higher affinity than prednisolone for the glucocorticoid receptor (Mollmann et al., 1995). Knowledge of the relative receptor affinities and prednisolone IC50,Free allows for calculation of a 0.3 ng/ml value for betamethasone IC50,Free (Mager and Jusko, 2002). Extrapolated maternal/fetal profiles for the two formulations were compared to this IC50,Free toxicity threshold to evaluate the two formulations. The longer the concentrations stay above the toxicity threshold, the greater is the potential for adverse effects.
Table 1.
Correction factors needed to make human extrapolations
| Scaling to the Clinical Dose |
Fetal to Maternal Gradient |
Plasma Free Fraction |
Combined Correction Factor |
|
|---|---|---|---|---|
| Maternal Circulation | ||||
| Phosphate/acetate formulation | 0.34a | N/A | 0.4b | 0.14 |
| Phosphate formulation | 0.68c | N/A | 0.4b | 0.28 |
| Fetal Circulation | ||||
| Phosphate/acetate formulation | 0.34a | 0.28d | 0.59b | 0.06 |
| Phosphate formulation | 0.68c | 0.28d | 0.59b | 0.11 |
N/A, not applicable.
Ratio of the clinical dose (0.17 mg/kg) to the studied dose (0.5 mg/kg).
Ratio of the clinical dose (0.17 mg/kg) to the studied dose (0.25 mg/kg).
The dissociation constant for the human fetal lung glucocorticoid receptor was chosen as an efficacy threshold. The evidence supporting corticosteroid-mediated induction of fetal lung maturation via the glucocorticoid cytosolic receptor and the utility of its dissociation constant have been reviewed (Ballard and Ballard, 1995). Most of the data come from experiments performed with fetal lung explant cultures. Fetal lung cells have been shown to possess glucocorticoid receptors, and the effects seen with glucocorticoids are reversible, indicating reversible binding of steroids to their receptors. Induction experiments show considerable delay in peak effects, (24 and 48 h for mRNA and protein up-regulation), which is in accordance with transcription and translation delays that occur after receptor binding. Induction effects have been observed only upon exposure to glucocorticoids and not when cells were treated with androgens, estrogens, or progestins. Most importantly, half-maximal induction of various molecular markers of fetal lung maturation occurs at concentrations that are similar to the dissociation constant values for receptor binding of glucocorticoids. We therefore chose the dissociation constant for the human fetal lung glucocorticoid receptor as the efficacy threshold. A dissociation constant of 5 nM for dexamethasone is available in the literature (Gonzales et al., 1986). Dexamethasone has 1.7-fold higher affinity than betamethasone for binding to the glucocorticoid receptor in the human lung (Mollmann et al., 1995). This information allows calculation of a dissociation constant value of 8.5 nM (3 ng/ml) for betamethasone. Extrapolated maternal/fetal profiles for the two formulations were compared to this efficacy threshold to discern which of the two formulations may have a better efficacy profile. The longer the concentrations stay above the efficacy threshold, the greater is the potential for producing fetal lung maturation. Extrapolated free betamethasone concentrations in fetal plasma can be directly compared to the dissociation constant for the receptor residing intracellularly in the lung because the lipophilicity of the steroid allows ready passage across cellular membranes.
Results
LC/MS/MS Analysis for Betamethasone
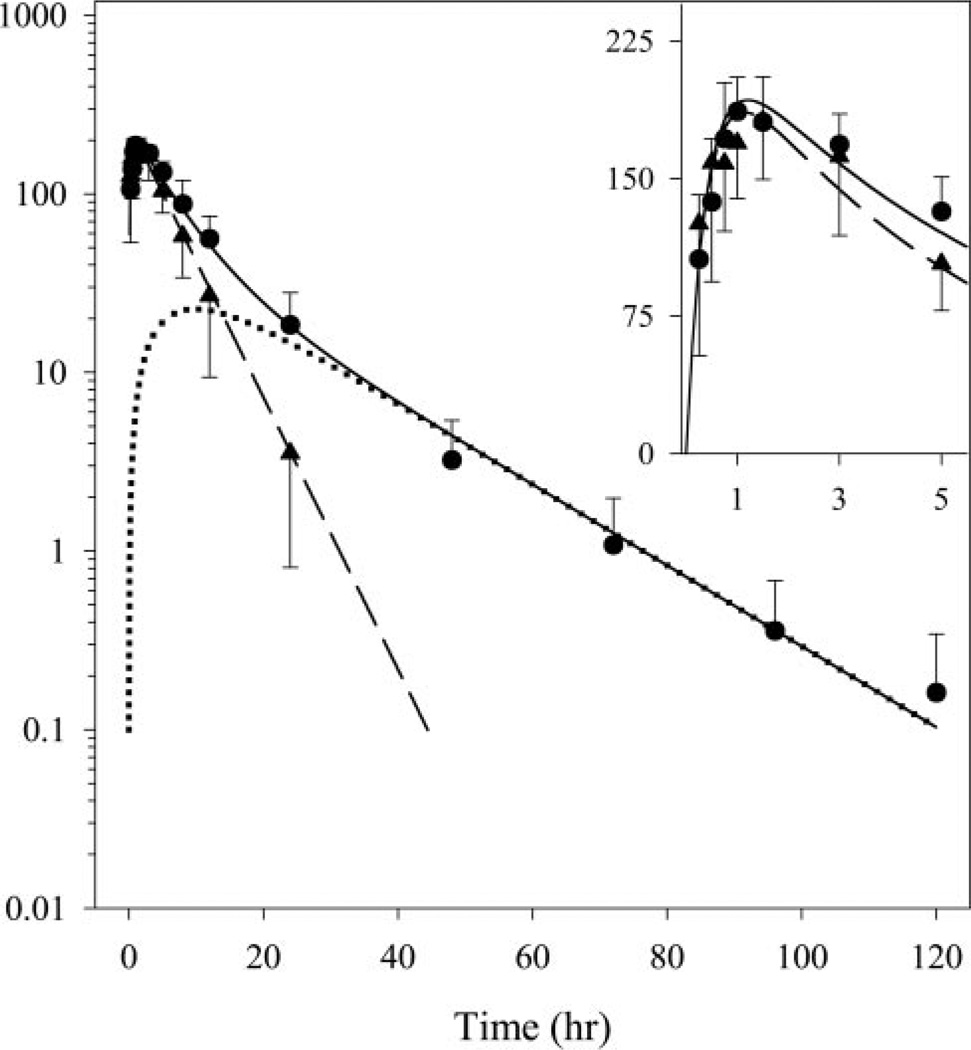
The enhanced sensitivity of the LC/MS/MS assay and the high doses used in the study allowed characterization of betamethasone pharmacokinetics for 24 h for the phosphate and for 5 days for the phosphate/acetate formulations (Fig. 2). The assay was adapted from a method described for rat plasma samples (Tamvakopoulos et al., 2002). An important change was made wherein the assay was scaled up to make it suitable for plasma samples from sheep. Tamvakopoulos et al. (2002) measured betamethasone in small-volume rat plasma samples and hence used a 96-well miniaturized HLB Oasis extraction format. Sample volume was not a limitation in our study, and we wished to measure extremely low drug concentrations. We therefore used the HLB Oasis cartridge format for extracting plasma samples, which involved a 10-fold larger sample volume (500 versus 50 µl). By using a larger sample volume, we were able to reduce the assay lower limit of quantification of 2 ng/ml, reported by Tamvakopoulos et al. (2002), to 0.1 ng/ml. During the preparation of this article, two other methods describing LC/MS/MS analysis of betamethasone in plasma were published (Taylor et al., 2004; Luo et al., 2005). These methods monitor different daughter ions in tandem mass spectrometry for betamethasone and make use of longer liquid-liquid extraction-based sample preparation methods. However, both these methods are similar to our assay in terms of assay sensitivity, accuracy, precision, specificity, and quantifiable concentration range.
Fig. 2.
Results of the pharmacokinetic study, with data points and standard deviation bars representing mean betamethasone concentrations in plasma from four animals after administration of betamethasone phosphate (▲) and the dual formulation (●). Solid and dashed curves are profiles fitted simultaneously to the phosphate and dual formulation data. The dotted curve represents the deduced betamethasone profile arising from the acetate component of the dual formulation. Inset, betamethasone concentrations and fitted curves on a linear scale for the first 5 h.
Betamethasone Pharmacokinetic Profiles
The mean betamethasone concentration profiles produced by the two formulations and the fitted curves are shown in Fig. 2. The profiles reported here, unlike all the sheep profiles summarized in a recent meta-analysis (Samtani et al., 2004a), show a distinct absorption/formation phase. The latter is indicative of lack of sample instability artifacts, which were prevented by addition of enzyme inhibitors. Both formulations produced a peak concentration of about 180 ng/ml at 1 to 1.5 h, which reflects the fast input from the phosphate prodrug (Table 2: kaBMp half-life of 0.3 h). Whereas the phosphate formulation profile appeared like a traditional Bateman function with a terminal half-life of 4 h, the phosphate/acetate formulation produced a biexponential decline with a shallow terminal half-life of 14 h. The betamethasone profile emerging from the acetate prodrug was obtained by simple subtraction of the two fitted curves and is depicted in Fig. 2. The longer terminal half-life of the dual prodrug formulation can be attributed to flip-flop kinetics, in which the slow decline in concentrations is reflective of the delayed sustained release of betamethasone from the intramuscular site. The lack of a distribution phase in the phosphate formulation profile suggests that the distribution process for betamethasone is either rapid or obscured by the formation/absorption phase. This pattern justifies the one-compartment model depicted for betamethasone in Fig. 1. The model-fitted curves in Fig. 2 indicate that the proposed models well captured the betamethasone pharmacokinetics. The estimated pharmacokinetic parameters are listed in Table 2, and the <15% coefficient of variation for all fitted parameters is indicative of excellent model performance.
TABLE 2.
Estimated betamethasone pharmacokinetic parameters after administration of two prodrug formulations
| Parameter | Estimate | CV (%)a |
|---|---|---|
| kaBMp (1/day) | 60.6 | 9.19 |
| kaBma (1/day) | 1.25 | 5.36 |
| Vc/FBMp (l/kg) | 1.10 | 3.57 |
| Vc/FBMa (l/kg) | 1.77 | 12.2 |
| kel (1/day) | 4.21 | 3.72 |
| t1/2 kaBMp (h) | 0.275b | 9.19 |
| t1/2 kaBMa (h) | 13.3b | 5.36 |
| t1/2 kel (h) | 3.95b | 3.72 |
| % FBMa/FBMp | 62.3b | 12.1 |
Coefficient of variation of the estimate, not reflective of inter-animal variability.
Secondary parameters.
Effect of Pregnancy on Betamethasone Pharmacokinetics
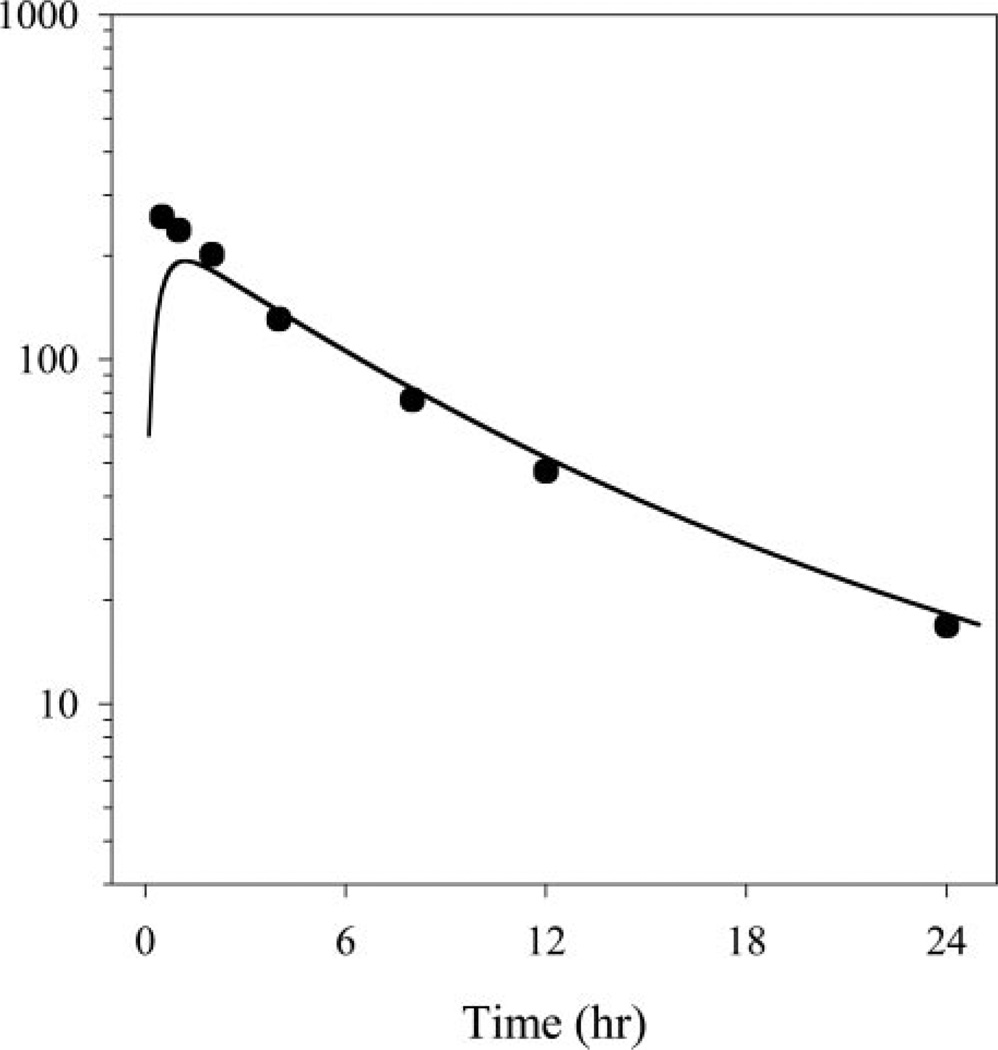
Figure 3 shows the fitted pharmacokinetic profile for the 0.5 mg/kg dual formulation from this study superimposed over the data from a recent meta-analysis depicting maternal pharmacokinetics from pregnant sheep at the same dose level. There is a lack of agreement at the early time points between the two datasets, which can be attributed to lack of sample stabilization in the pregnant animal data. Apart from the early disconnect in concentrations, there is reasonable agreement between the two datasets. This pharmacokinetic trait of similarity in betamethasone disposition between the pregnant and nonpregnant states has also been observed in humans (Benet et al., 1996). We have recently shown that sheep pharmacokinetic profiles for betamethasone in pregnant sheep are a good representation of human profiles in pregnancy (Samtani et al., 2004a). Minimal scaling of pharmacokinetics is required because of similarity in body weights. The resemblance between humans and sheep for betamethasone pharmacokinetics during the pregnant and nonpregnant states allows the use of sheep as an excellent animal model for anticipating betamethasone disposition during pregnancy in humans.
Fig. 3.
The fitted pharmacokinetic profile for the 0.5 mg/kg dual formulation profile from Fig. 2 superimposed over data from a recent meta-analysis (Samtani et al., 2004a) depicting maternal betamethasone pharmacokinetics from pregnant sheep over 24 h at the same dose level.
Extrapolated Human Profiles
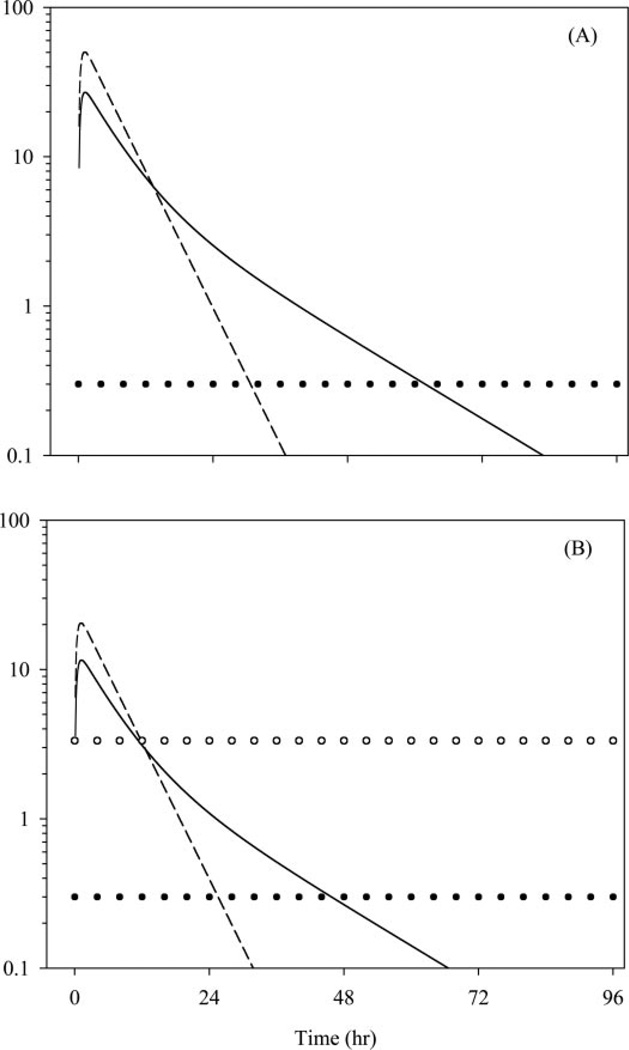
Extrapolations to the human situation are shown in Fig. 4, and they indicate that the two formulations given at equal doses will produce markedly different profiles in the maternal and fetal circulations. The 12-mg dose of betamethasone in the form of the phosphate prodrug would generate higher peak levels both in the mother and in the fetus and produce a steep decline in concentrations. In contrast, 12 mg of the dual formulation (8 mg of betamethasone phosphate equivalent to 6 mg of betamethasone and 6 mg of betamethasone acetate) would produce lower peak concentrations in the maternal and fetal circulations. However, maternal and fetal profiles would have a prolonged terminal half-life because of the sustained-release nature of the acetate prodrug.
Fig. 4.
Extrapolated human profiles for unbound maternal (A) and fetal (B) betamethasone concentrations. Solid and dashed curves represent profiles for the dual and phosphate formulations. Horizontal lines depict the toxicity threshold (solid circles) and the efficacy threshold (open circles).
The delayed release property of the dual formulation causes betamethasone concentrations to stay above the proposed toxicity threshold for a longer period in maternal and fetal plasma, and this sustained exposure is shorter in the fetal than in the maternal circulation. This is because restricted placental access of betamethasone produces lower peak concentrations in the fetus, and hence, it takes a shorter duration of time for betamethasone levels to fall below the toxicity threshold.
Finally, the time of exposure to therapeutic betamethasone concentrations in the fetal circulation is similar. Both formulations produce profiles that cross the therapeutic threshold at approximately the same time.
Discussion
Choice of LC/MS/MS for Analysis
Commonly used high-performance liquid chromatography assays for corticosteroids have a lower limit of quantitation of 10 ng/ml (Jusko et al., 1994). Other methods based on gas chromatography/mass spectrometry and fluorescence detection, although offering highly specific and sensitive analysis, require derivatization (Frerichs and Tornatore, 2004). Bioanalytical methods based on LC/MS/MS represent the most specific and sensitive methods for steroid analysis (Lai et al., 2002). This technique coupled with solid-phase extraction combines the attributes of rapidity, easy processing, accuracy, specificity, sensitivity, and precision without the need for sample derivatization. We therefore assayed betamethasone in sheep plasma using a solid-phase extraction LC/MS/MS method, which allowed measurement of slow betamethasone release from the acetate prodrug.
Release Rates of Active Steroid by Betamethasone Prodrugs
It is generally accepted that the conversion of the acetate and phosphate prodrugs to the active corticosteroid is a relatively fast process. Studies in rodents have shown that the acetate prodrug administered intravenously (dissolved in dimethyl sulfoxide containing saline) produces corticosteroid pharmacokinetics that are identical to those produced by an intravenously administered phosphate prodrug (Ogiso et al., 1987). The inability of the conversion process to serve as a rate-limiting step is not surprising because activating enzymes such as phosphatases and esterases are highly efficient and ubiquitous (Krise et al., 1999).
The release rate of betamethasone from the two prodrugs is controlled by their physicochemical properties. The phosphate prodrug, being highly ionized, has an aqueous solubility of 625 mg/ml (Harvey, 1975). It is injected as a solution and exhibits rapid absorption after intramuscular administration. Consequently, the disposition processes controlling betamethasone pharmacokinetics dictate the terminal decline in concentrations after injection of the phosphate prodrug. In contrast, the acetate prodrug is highly hydrophobic, has an aqueous solubility of 30 µg/ml (Kabasakalian et al., 1966), and is administered intramuscularly as a suspension. The >20,000-fold lower solubility creates dissolution rate-limited absorption of the acetate prodrug. It has to first dissolve in the fluids of the intercellular space of muscle fibers before it can diffuse into the vascular space (Hirano et al., 1981). For practically water-insoluble molecules in vitro solubility is directly correlated to in vivo absorption after intramuscular injection of an aqueous suspension (Zuidema et al., 1994). The solubilization of the suspension is often the slowest event among the absorption, distribution, metabolism, and excretion processes after intramuscular injection. The rate-limiting solubilization process is reflected in the terminal slope of the pharmacokinetic profile leading to flip-flop kinetics. Furthermore, interspecies differences in the terminal half-life are not expected since the dissolution process at the injection depot governs the terminal decline (Zuidema et al., 1994). Thus, the long half-life observed for betamethasone in this study after injection of the dual formulation may also occur in humans. We believe that the long half-life has not been detected previously after intramuscular administration because of assay and sampling limitations. Furthermore, the acetate prodrug is also only 62% bioavailable as compared to the phosphate prodrug (Table 2: FBMa/FBMp = 62.3%). This is not surprising because highly lipophilic compounds produce biphasic absorption after intramuscular injection, wherein the second slow phase is exceptionally difficult to capture, leading to the conclusion of incomplete bioavailability (Zuidema et al., 1994). This slow second phase is thought to produce a “hangover effect,” which leads to drug levels that are probably not therapeutically useful and may be a source of unwanted side effects (Zuidema et al., 1994). The possibility even exists that some drug continues to be absorbed beyond 120 h with a third phase of release (Fig. 2).
Biexponential Decline Following the Dual Formulation
Multiexponential decline patterns for betamethasone can be observed due to various reasons. Possibilities include 1) spurious overestimation of concentrations due to sample instability (Samtani et al., 2004b), 2) tissue distribution, or 3) dual betamethasone input. It is often assumed that the acetate prodrug does not release betamethasone, and the biexponential profile has been attributed to tissue distribution (Samtani et al., 2004a). However, Fig. 2 clearly indicates that the acetate does have sustained-release properties and the phosphate prodrug profile lacks an obvious distribution phase. Furthermore, we prevented artifactual overestimation of concentrations by sample stabilization, and therefore, the most probable explanation for betamethasone multiexponentiality is dual steroid input. This finding probably indicates that estimates of tissue distribution volumes and clearances reported in the literature need to be interpreted with caution because they may have been estimated inaccurately due to model misspecification.
Observation of a biexponential decline pattern is a typical feature of analytes with dual input. A classic example is metabolite kinetics observed after oral administration of a drug exhibiting high oral first-pass effect (Rowland and Tozer, 1995). The mathematical rationale behind the biexponential decline can be readily understood by analyzing eq. 2 for the dual formulation. Integrating eq. 2 gives the following explicit equation:
| (4) |
The explicit equation is merely a sum of two Bateman functions, where one function has kel as the rate-limiting process, whereas the other has kaBMa as the rate-limiting step. The two functions are displaced in time because of different rate-limiting processes, and superimposition of two such functions produces a curve with biexponential decline.
Reconciling Different Beliefs about Acetate Prodrug Release
Studies that have concluded that the acetate prodrug does not release betamethasone were short-duration studies that spanned 8 to 10 h (Petersen et al., 1984). Figure 2 demonstrates that, during this early time period, the pharmacokinetic profiles from the two formulations are virtually superimposable. Due to the depot release nature of the acetate prodrug, it delivers very little betamethasone during this early period. The minuscule amount of betamethasone released during the early phase and the inability to measure the low concentrations of betamethasone beyond 8 to 10 h has led to erroneous conclusions about the acetate prodrug. In contrast, studies conducted by Ballard et al. (1975) have examined the clinical dosing regimen of two doses of 12-mg betamethasone phosphate/acetate 24 h apart. Based on the half-life obtained from this study and information from betamethasone phosphate studies in nonpregnant subjects, Ballard has stated that the dual formulation does have sustained-release properties (Ballard, 1986; Ballard and Ballard, 1995). The terminal half-life of 6 h reported by Ballard is the widely accepted half-life for betamethasone after administration of the dual formulation (Padbury et al., 1996; Dudley et al., 2003). Although Ballard made an insightful and accurate conclusion about the dual-release properties of the phosphate/acetate formulation, the commonly accepted half-life is probably incorrect. Based on the data in Fig. 2, it is obvious that the true terminal decline phase of betamethasone from the acetate prodrug will be missed if the profile is followed for any duration less than 2 to 3 days. In light of this new finding, dosing recommendations for betamethasone formulations need to be reassessed. This finding is particularly important because, in the United States, the only injectable form of betamethasone recommended and available for antenatal use is the dual formulation. Betamethasone sodium phosphate injection now appears in the “discontinued” section of Food and Drug Administration’s Orange Book because Schering-Plough discontinued the manufacture of this product in May 2002. Furthermore, many clinicians have adopted the practice of giving multiple doses (as many as 11 repeat courses) of betamethasone to enhance fetal lung maturation (Andrews and Matthews, 2003). The prolonged half-life for betamethasone from the intramuscular depot combined with multiple dosing can have accumulative and unfavorable effects in the mother and fetus, and this will be discussed below.
Implications of a Prolonged Half-Life on Fetal and Maternal Health
Results from Fig. 4 indicate that both betamethasone formulations produce similar early exposures, but the dual formulation could have a lower safety index. This is supported by the fact that efficacy of both formulations has been demonstrated in pregnancy trials. However, a recently conducted meta-analysis (Jobe and Soll, 2004) has shown that betamethasone use in pregnancy might be associated with a higher incidence of maternal infections as compared with dexamethasone. The majority of the betamethasone trials in the meta-analysis used the dual formulation, whereas dexamethasone was exclusively used as the phosphate prodrug. The mother serves as a delivery mode and reservoir for the steroid and is exposed to betamethasone levels 3 times higher than that in the fetus. The practice of exposing the parturient mother to steroid levels that are of no therapeutic benefit to her and probably lead to adverse effects is unfortunate. Reassessment of use of this dual-formulation regimen during pregnancy is warranted.
Several assumptions have been made in arriving at the conclusion regarding the reassessment of corticosteroid dosing regimens during pregnancy. These conclusions need to be supported by additional long-duration experimental data on corticosteroid pharmacokinetics, adrenal suppression, and fetal drug exposure from different formulations during pregnancy. Corticosteroids have been used for precocious induction of fetal lung maturation for over 30 years. However, the pharmacokinetic, pharmacodynamic, and toxicodynamic properties of corticosteroids in pregnancy are poorly understood and can be improved by well designed mechanistic studies using modern analytical tools.
Acknowledgments
We thank Donna Ruszaj for helpful discussions and technical assistance with LC/MS/MS assay development.
This study was supported by Grants GM 24211 and HD 21350 from the National Institutes of Health and a predoctoral fellowship for M.N.S. from Merck. The liquid chromatograph/tandem mass spectrometer was obtained through a shared instrumentation grant (S10RR14573) from the National Center for Research Resources, National Institutes of Health.
ABBREVIATIONS
- LC/MS/MS
liquid chromatography/tandem mass spectrometry
- QC
quality control
- BMp, BMa, and BMp/a
betamethasone phosphate prodrug, acetate prodrug, and dual phosphate/acetate formulation-related variables
- CBMp and CBMp/a
betamethasone concentrations
- kaBMp and kaBMa
hybrid first-order rate constants
- DoseBMp and DoseBMa
doses of prodrugs in terms of betamethasone equivalents
- Vc/FBMp and Vc/FBMa
apparent volumes of distribution
- FBMa and FBMp
bioavailability terms
- FBMa/FBMp
relative bioavailability
- Y(t)
model output function
- IC50,Free
free drug level causing 50% inhibition of cortisol synthesis rate
References
- Andrews MH, Matthews SG. Antenatal glucocorticoids: Is there cause for concern? Fetal and Maternal Medicine Review. 2003;14:329–354. [Google Scholar]
- Ballard PL. Hormones and lung maturation. Monogr Endocrinol. 1986;28:173–196. [PubMed] [Google Scholar]
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Investig. 1975;56:1548–1554. doi: 10.1172/JCI108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet LZ, Oie S, Schwartz JB. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill Companies; 1996. pp. 1707–1792. [Google Scholar]
- D’Argenio DZ, Schumitzky A. ADAPT II User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles: Biomedical Simulations Resource; 1997. [Google Scholar]
- Ding J, Neue UD. A new approach to the effective preparation of plasma samples for rapid drug quantitation using on-line solid phase extraction mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:2151–2159. doi: 10.1002/(SICI)1097-0231(19991115)13:21<2151::AID-RCM767>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Dudley DJ, Waters TP, Nathanielsz PW. Current status of single-course antenatal steroid therapy. Clin Obstet Gynecol. 2003;46:132–149. doi: 10.1097/00003081-200303000-00018. [DOI] [PubMed] [Google Scholar]
- Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. J Matern Fetal Med. 1997;6:309–313. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Frerichs VA, Tornatore KM. Determination of the glucocorticoids prednisone, prednisolone, dexamethasone and cortisol in human serum using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:329–338. doi: 10.1016/j.jchromb.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. Br J Obstet Gynaecol. 1989;96:401–410. doi: 10.1111/j.1471-0528.1989.tb02413.x. [DOI] [PubMed] [Google Scholar]
- Gonzales LW, Ballard PL, Ertsey R, Williams MC. Glucocorticoids and thyroid hormones stimulate biochemical and morphological differentiation of human fetal lung in organ culture. J Clin Endocrinol Metab. 1986;62:678–691. doi: 10.1210/jcem-62-4-678. [DOI] [PubMed] [Google Scholar]
- Harvey SC. Hormones. In: Hoover JE, editor. Remington’s Pharmaceutical Sciences. Easton, PA: Mack Publishing Company; 1975. pp. 879–934. [Google Scholar]
- Hirano K, Ichihashi T, Yamada H. Studies on the absorption of practically water-insoluble drugs following injection. II. Intramuscular absorption from aqueous suspensions in rats. Chem Pharm Bull (Tokyo) 1981;29:817–827. doi: 10.1248/cpb.29.817. [DOI] [PubMed] [Google Scholar]
- Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol. 2004;190:878–881. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Jusko WJ, Pyszczynski NA, Bushway MS, D’Ambrosio R, Mis SM. Fifteen years of operation of a high-performance liquid chromatographic assay for prednisolone, cortisol and prednisone in plasma. J Chromatogr B Biomed Appl. 1994;658:47–54. doi: 10.1016/0378-4347(94)00218-5. [DOI] [PubMed] [Google Scholar]
- Kabasakalian P, Britt E, Yudis MD. Solubility of some steroids in water. J Pharm Sci. 1966;55:642. doi: 10.1002/jps.2600550625. [DOI] [PubMed] [Google Scholar]
- Krise JP, Narisawa S, Stella VJ. A novel prodrug approach for tertiary amines. 2. Physicochemical and in vitro enzymatic evaluation of selected N-phosphonooxymethyl prodrugs. J Pharm Sci. 1999;88:922–927. doi: 10.1021/js9803813. [DOI] [PubMed] [Google Scholar]
- Lai CC, Tsai CH, Tsai FJ, Wu JY, Lin WD, Lee CC. Rapid screening assay of congenital adrenal hyperplasia by measuring 17 alpha-hydroxyprogesterone with high-performance liquid chromatography/electrospray ionization tandem mass spectrometry from dried blood spots. J Clin Lab Anal. 2002;16:20–25. doi: 10.1002/jcla.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- Luo Y, Uboh CE, Soma LR, Guan F, Rudy JA, Tsang DS. Resolution, quantification and confirmation of betamethasone and dexamethasone in equine plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:825–832. doi: 10.1002/rcm.1851. [DOI] [PubMed] [Google Scholar]
- Mager DE, Jusko WJ. Quantitative structure-pharmacokinetic/pharmacodynamic relationships of corticosteroids in man. J Pharm Sci. 2002;91:2441–2451. doi: 10.1002/jps.10231. [DOI] [PubMed] [Google Scholar]
- Makin HLJ, Honour JW, Shackleton CHL. General methods of steroid analysis. Part 1. Extraction, purification and measurement of steroids by HPLC, GLC and mass spectrometry. In: Makin HLJ, Gower DB, Kirk BN, editors. Steroid Analysis. Glasgow: Blackie & Son, Ltd.; 1995. pp. 114–184. [Google Scholar]
- Mollmann H, Balbach S, Hochhaus G, Barth J, Derendorf H. Pharmacokinetic-pharmacodynamic correlations of corticosteroids. In: Derendorf H, Hochhaus G, editors. Handbook of Pharmacokinetic/Pharmacodynamic Correlation. Boca Raton, FL: CRC Press Inc.; 1995. pp. 323–361. [Google Scholar]
- Moss TJ, Sloboda DM, Gurrin LC, Harding R, Challis JR, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol. 2001;281:R960–R970. doi: 10.1152/ajpregu.2001.281.3.R960. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Panel. NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. J Am Med Assoc. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- Ogiso T, Ito Y, Iwaki M, Atago H, Tanaka C, Maniwa N, Ishida S. Percutaneous absorption of dexamethasone acetate and palmitate and the plasma concentration. Chem Pharm Bull (Tokyo) 1987;35:4263–4270. doi: 10.1248/cpb.35.4263. [DOI] [PubMed] [Google Scholar]
- Padbury JF, Ervin MG, Polk DH. Extrapulmonary effects of antenatally administered steroids. J Pediatr. 1996;128:167–172. doi: 10.1016/s0022-3476(96)70384-0. [DOI] [PubMed] [Google Scholar]
- Petersen MC, Ashley JJ, McBride WG, Nation RL. Disposition of betamethasone in parturient women after intramuscular administration. Br J Clin Pharmacol. 1984;18:383–392. doi: 10.1111/j.1365-2125.1984.tb02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, Collier CB, Ashley JJ, McBride WG, Nation RL. Disposition of betamethasone in parturient women after intravenous administration. Eur J Clin Pharmacol. 1983;25:803–810. doi: 10.1007/BF00542524. [DOI] [PubMed] [Google Scholar]
- Petersen MC, Nation RL, Ashley JJ. Simultaneous determination of betamethasone, betamethasone acetate and hydrocortisone in biological fluids using high-performance liquid chromatography. J Chromatogr. 1980;183:131–139. doi: 10.1016/s0378-4347(00)81686-2. [DOI] [PubMed] [Google Scholar]
- Rodger MA, King L. Drawing up and administering intramuscular injections: a review of the literature. J Adv Nurs. 2000;31:574–582. doi: 10.1046/j.1365-2648.2000.01312.x. [DOI] [PubMed] [Google Scholar]
- Rowland M, Tozer TN. Clinical Pharmacokinetics: Concept and Applications. Baltimore: Lippincott Williams & Wilkins; 1995. [Google Scholar]
- Samtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Area/moment and compartmental modeling of pharmacokinetics during pregnancy: applications to maternal/fetal exposures to corticosteroids in sheep and rats. Pharm Res (NY) 2004a;21:2279–2292. doi: 10.1007/s11095-004-7681-7. [DOI] [PubMed] [Google Scholar]
- Samtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Stabilization and HPLC analysis of betamethasone sodium phosphate in plasma. J Pharm Sci. 2004b;93:726–732. doi: 10.1002/jps.10577. [DOI] [PubMed] [Google Scholar]
- Tamvakopoulos CS, Neugebauer JM, Donnelly M, Griffin PR. Analysis of betamethasone in rat plasma using automated solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. Determination of plasma concentrations in rat following oral and intravenous administration. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;776:161–168. doi: 10.1016/s1570-0232(02)00271-4. [DOI] [PubMed] [Google Scholar]
- Taylor RL, Grebe SK, Singh RJ. Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin Chem. 2004;50:2345–2352. doi: 10.1373/clinchem.2004.033605. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H, Nishikawa M, Katagi M, Tsuchihashi H. Adsorption and stability of suxamethonium and its major hydrolysis product succinylmonocholine using liquid chromatography- electrospray ionization mass spectrometry. J Health Sci. 2003;49:285–291. [Google Scholar]
- Wald JA, Law RM, Ludwig EA, Sloan RR, Middleton E, Jr, Jusko WJ. Evaluation of dose-related pharmacokinetics and pharmacodynamics of prednisolone in man. J Pharmacokinet Biopharm. 1992;20:567–589. doi: 10.1007/BF01064420. [DOI] [PubMed] [Google Scholar]
- Zuidema J, Kadir F, Titulaer HAC, Oussoren C. Release and absorption rates of intramuscularly and subcutaneously injected pharmaceuticals (II) Int J Pharm. 1994;105:189–207. [Google Scholar]