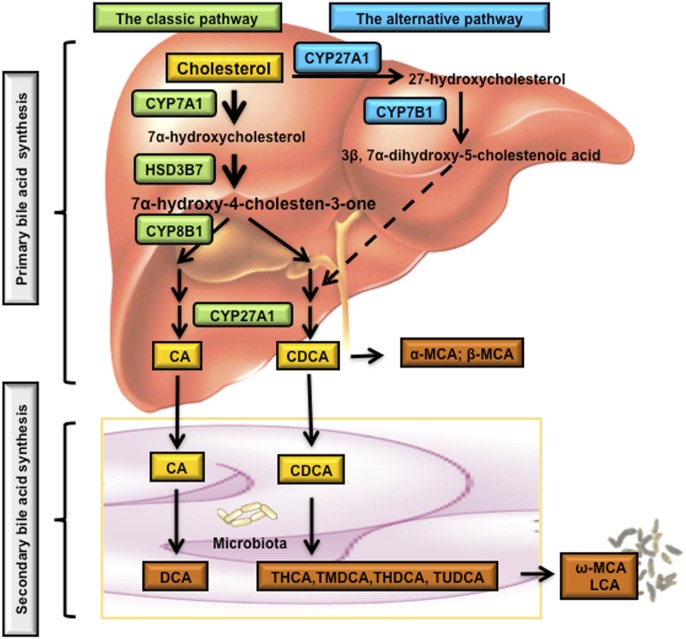
Fig. 2.
Bile acid biosynthetic pathways. Two major bile acid biosynthetic pathways are shown. In the classic pathway, cholesterol is converted to 7α-hydroxycholesterol by the rate-limiting enzyme CYP7A1, which is located in the ER. The 3β-hydroxysteroid dehydrogenase (3βHSD, HSD3B7) converts 7α-hydroxycholesterol to 7α-hydroxy-4-cholesten-3-one (C4), which is converted to 7α,12α-dihydroxy-4-cholesten-3-one by a sterol 12α-hydroxylase (CYP8B1) leading to synthesis of CA. Without 12α-hydroxylation by CYP8B1, C4 is eventually converted to CDCA. The mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes the steroid side chain oxidation in both CA and CDCA synthesis. In the alternative pathway, cholesterol is first converted to 27-hydroxycholesterol by CYP27A1. Oxysterol 7α- hydroxylase (CYP7B1) catalyzes hydroxylation of 27-hydroxycholesterol to 3β,7α-dihydroxy-5-cholestenoic acid, which eventually is converted to CDCA. Oxysterol 7α-hydroxylase (CYP7B1) is nonspecific and can also catalyze hydroxylation of 25-hydroxycholesterol to 5-cholesten-3β,7α,25-triol. In the large intestine, bacterial 7α-dehydroxylase removes a hydroxyl group from C-7 and converts CA to DCA and CDCA to LCA. In mouse liver, most of CDCA is converted to α- and β-MCA. In the intestine, bacterial 7α-dehydroxylase activity convers CA and CDCA to DCA and LCA, respectively. CYP3A1 and epimerase also convert CDCA to the secondary bile acids, including THCA, TMDCA, ω-MCA, THDCA, and TUDCA. LCA and ω-MCA are excreted into feces. THCA, taurohyocholic acid; THDCA, taurohyodeoxycholic acid; TMDCA, tauromurideoxycholic acid.