Fig. 3. Putative membrane binding footprint of epsin ENTH domain.
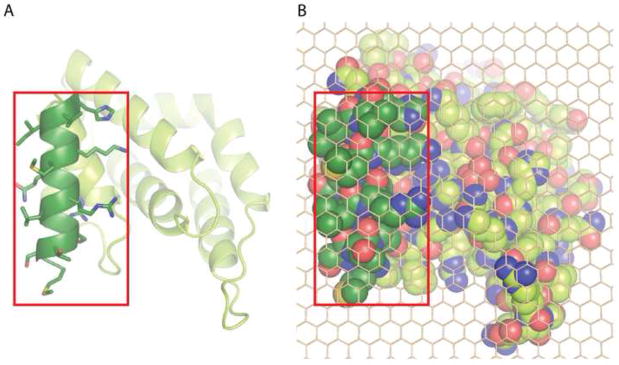
A. Membrane interaction surface of epsin1 ENTH domain viewed from the membrane. The amphipathic helix, which inserts into the membrane is shown in dark green with amino acid side chains included.
B. Space filled representation of (A) with side chains included for the whole protein, overlaid with a carbon atom lattice (to facilitate area calculations), showing that the amphipathic helix occupies approximately 1/3rd of the total projection area.
