Figure 2.
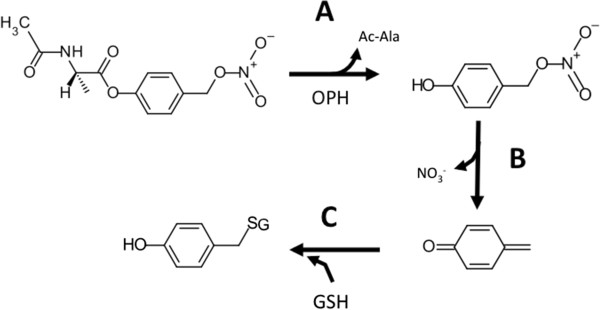
Mechanism of N-acetylalaninate prodrug activation by OPH and subsequent depletion of glutathione. A) The ester bond of the prodrug is cleaved by the esterase activity of oxidized protein hydrolase (OPH) releasing acetylalaninate (Ac-Ala) and a (4-hydroxyphenyl)methyl nitrate intermediate. B) The intermediate quickly undergoes elimination releasing NO3 - and forming a reactive quinone methide (QM). C) The QM rapidly reacts with the thiol group of reduced glutathione (GSH) in a Michael addition leaving GSH unavailable to participate in cellular redox reactions.
