Figure 1.
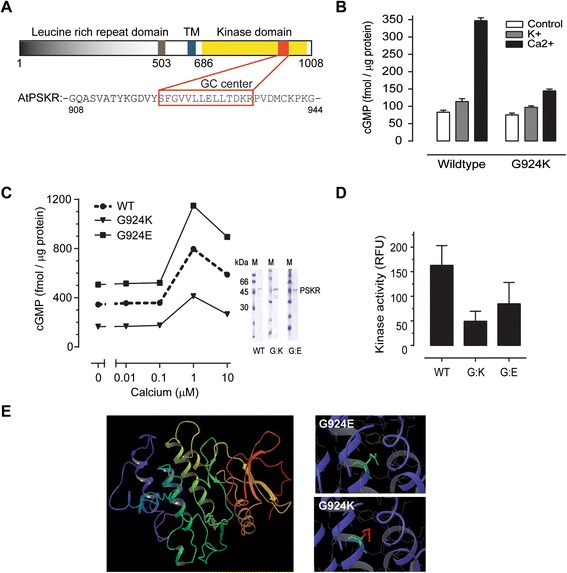
Effect of calcium on guanylate cyclase activity of PSKR1. A Schematic diagram of PSKR1 featuring the sequence motif of the guanylate cyclase catalytic centre and the immediately surrounding amino acids (908–944). TM refers to the transmembrane domain and the ligand binding domain occurs in the extracellular region from residues 503 to 517. B Effect of cations on guanylate cyclase activity of PSKR1. The cytoplasmic domain of PSKR1 (residues 683 to 1008) was expressed as either wild-type His-tagged SUMO-fused protein or the mutant protein (G924K) prepared as previously described [6]. Calcium significantly enhanced guanylate cyclase activity and the G924K mutant had significantly less activity than the wild-type (mean ± s.e.m., n = 3; P < 0.0001 two-way ANOVA, Sidak’s multiple comparison test). C The effect of calcium on the guanylate cyclase activity of wild-type and mutant (G924K and G924E) PSKR1 (residues 686 to 1008) was measured at increasing calcium concentrations buffered with EGTA and Mg2+. The curves and all the treatments at 1 and 10 μM are significantly different (mean ± s.e.m. (error bars within symbol), n = 3 independent experiments; P < 0.0001 two-way ANOVA, Tukey-Kramer multiple comparison test). The purified wild-type and mutant PSKR1 (residues 686–1008) molecules (1 μg) were analysed by SDS-PAGE. D The kinase activity of the G924K or G924E mutants (residues 686–1008) determined at 8 nM free calcium were not significantly different to the wild-type (mean ± s.e.m., n = 3 independent experiments; P = 0.1532 one-way ANOVA). E Homology model of the cytoplasmic domain of PSKR1 was developed based on tomato resistance protein Pto. The homology model was mutated in the guanylate cyclase domain to show the effect of G924E (tolerated) and G924K (steric hindrance). The red colour associated with the lysine residue indicates steric hindrance and strain.
