Abstract
Secondary sclerosing cholangitis is a rare condition caused by disorders directly damaging the biliary tree. We present a case of a 34-year-old man with no pre-existing hepatobiliary disease who developed significant cholestasis and subsequent cholangitis while in the intensive care unit for multiorgan failure secondary to H1N1 influenza A (swine flu). After discharge from the intensive care unit, jaundice, fevers, abdominal pain, pruritus and ongoing cholestasis persisted, consistent with recurrent cholangitis. Secondary sclerosing cholangitis was confirmed by liver biopsy and endoscopic retrograde cholangiopancreatography. This is a case of the recently described syndrome of secondary sclerosing cholangitis following critical illness, with associated severe hypoxic and ischaemic injury. He subsequently developed recognised complications of sclerosing cholangitis, including fat-soluble vitamin deficiencies, recurrent cholangitis and liver fibrosis. To the best of our knowledge, this is the first reported case of secondary sclerosing cholangitis following critical illness in the UK.
Background
Primary sclerosing cholangitis (PSC) is a rare idiopathic condition with immunopathogenic mechanisms where there is chronic progressive destruction of the biliary tree. The majority of patients are found to have concomitant ulcerative colitis (UC). Secondary sclerosing cholangitis (SSC) is clinically comparable to PSC, but is caused by specific processes which directly damage the biliary tree.1 Examples include recurrent choledocholithiasis, abdominal trauma (including secondary to abdominal surgery), recurrent pancreatitis (including autoimmune pancreatitis), bile duct malignancy, congenital bile duct abnormalities such as Caroli's disease, biliary ischaemia from hepatic arterial occlusion post liver-transplantation, chemotherapeutic agents (eg, 5-fluorouracil), scolicides instilled into the biliary tree inadvertently during hydatid cyst therapy and AIDS-related cholangiopathy. The condition is progressive, leading to biliary fibrosis, recurrent cholangitis and ultimately cirrhosis. From the limited data available, median liver transplant-free survival is 72 months. Patients with SSC have a poorer prognosis than those with PSC.1
A new cause of SSC has been described during or following significant critical illness (SSC-CI) associated with severe respiratory insufficiency, vasopressor requirement and sepsis.1 2 To date, fewer than 100 cases of this condition are reported in the literature, the majority in Germany. The condition rapidly progresses to cirrhosis, frequently requiring liver transplantation for definitive management.3
Case presentation
A 34-year-old white man was referred to the hepatology team with jaundice, right upper quadrant abdominal pain and fevers 5 weeks after severe critical illness while still in the intensive care unit (ICU). His past medical history was unremarkable and he consumed on average three units of alcohol per week.
The primary presentation at his local hospital was a 7-day history of cough, dyspnoea and rigors. He had a fever of 38.1°C, severe desaturation at rest to 80% and tachycardia of 100 bpm. Mean arterial pressure was maintained at 100 mm Hg and urine output was poor at 20 mL/h despite fluid resuscitation. Chest auscultation demonstrated bilateral crepitations and chest x-ray confirmed bilateral infiltrates. Blood tests revealed elevated white cell count at 13.1×109/L, creatinine 276 µmol/L and arterial blood gas confirmed type II respiratory failure with acidosis (pH 7.22, PO2 7.8 kPa, PCO2 7.26 kPa). A transthoracic echocardiogram confirmed normal biventricular heart function and heart valves.
He was transferred to the ICU for management of multiorgan failure, intubated and ventilated and commenced on broad spectrum antibiotics. Oseltamivir (Tamiflu) was commenced as the respiratory virus molecular panel revealed H1N1 influenza A RNA (swine flu), which was further complicated by acute renal failure and severe acute respiratory distress syndrome on presentation. This prompted referral to our ICU within 48 h.
On arrival at our ICU, ventilation was initially switched to high frequency oscillation ventilation due to persistent hypoxia and hypercapnia and, on the fifth day, this was changed to extracorporeal membrane oxygenation (ECMO) assisted ventilation as acidosis and hypercapnia continued to progress. Oseltamivir was switched to zanamivir (Relenza) and rotational antibiotics were continued in view of ongoing fevers, with negative blood cultures. He received 19 days of inotropic support with noradrenaline, 22 days of assisted ECMO ventilation (and thereafter he was successfully extubated) and 28 days of renal replacement therapy with haemofiltration.
Thirty-two days after his transfer he complained of right upper quadrant abdominal pain, with ongoing fevers and deterioration in his liver function tests (table 1).
Table 1.
Progression of blood tests
| Test (reference range) | Presentation (December 2010) | Day 32 | March 2011 | November 2011 (before first ERCP) | July 2012 (peak liver dysfunction) | November 2013 |
|---|---|---|---|---|---|---|
| BIL (0–21 µmol/L) | 9 | 124 | 68 | 179 | 323 | 78 |
| ALP (35–129 IU/L) | 119 | 1057 | 2297 | 616 | 1048 | 477 |
| ALT (4–45 IU/L) | 112 | 247 | 171 | 229 | 248 | 333 |
| GGT (4–72 IU/L) | 655 | 1278 | 546 | 798 | 909 | 1070 |
| INR (0.8–1.2) | 0.94 | 1.12 | 1.1 | 1.2 | 1.2 | 1.0 |
| CR (59–104 µmol/L) | 269 | 134 | 78 | 65 | 71 | 72 |
| WBC (4–11×109) | 13.1 | 15.7 | 16.0 | 13.7 | 13.7 | 9.2 |
| CRP (0–4 mg/L) | 200 | 200 | 63 | 103 | 87 | 5 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; BIL, bilirubin; CR, creatinine; CRP, C-reactive protein; ERCP, endoscopic retrograde cholangiopancreatography; GGT, γ-glutamyl transpeptidase; INR, International normalised ratio; WBC, white blood cell count.
Investigations and treatment
Enterobacter was confirmed in blood cultures and chronic liver disease screen was negative (viral hepatitis serology for hepatitis B and C, autoimmune screen including perinuclear antineutrophil cytoplasmic antibodies, immunoglobulin levels, iron studies, α1-antitrypsin level and caeruloplasmin). Ultrasound of the abdomen was followed by magnetic resonance cholangiopancreatography (MRCP)/MRI of the liver (figure 1), which revealed hepatic and biliary abscesses, the largest in the right lobe at 2.8 cm. Minimal intrahepatic duct (IHD) dilation was seen, the common bile duct (CBD) was of normal calibre and no gallstones were present. Bile duct enhancement was normal excluding significant cholangitis. Antibiotics to treat the abscesses (rotation between gentamicin and ciprofloxacin, based on sensitivities from blood cultures) were commenced and the largest abscess was aspirated. Liver function tests improved (table 1) and repeat MRI liver confirmed radiological improvement.
Figure 1.
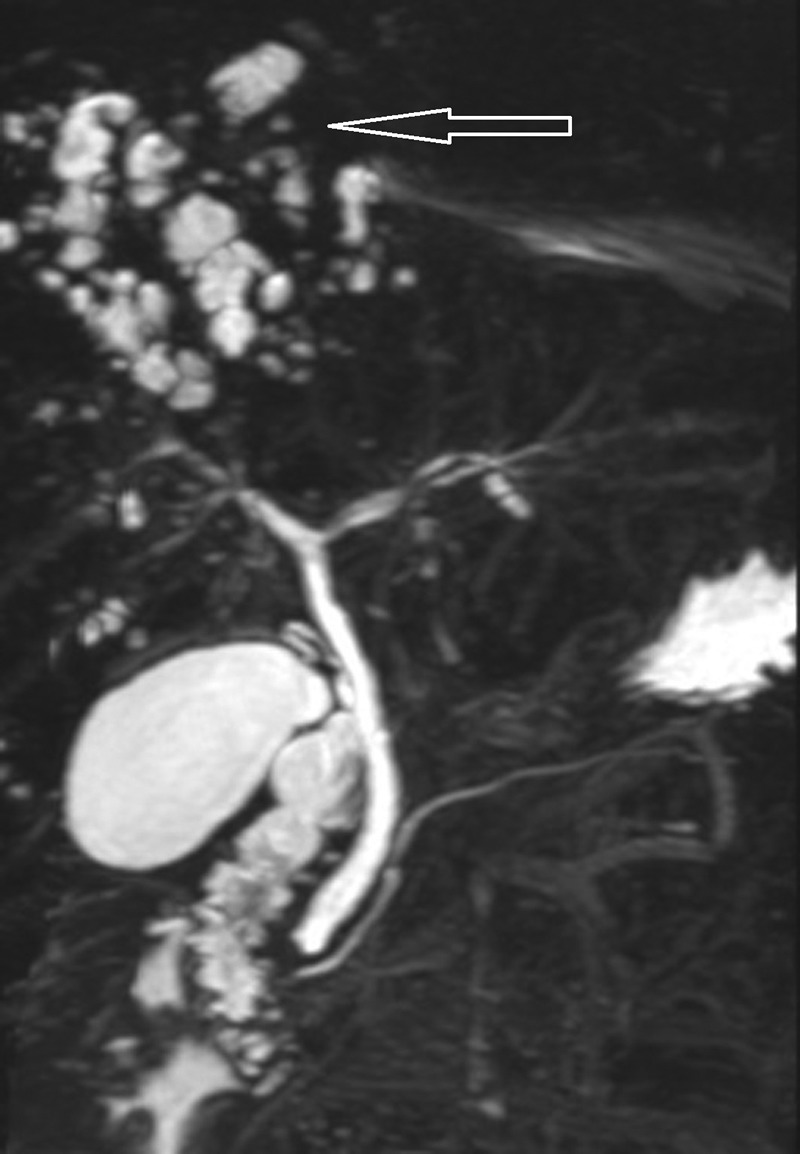
Magnetic resonance cholangiopancreatogram showing multiple high signal lesions in the liver in keeping with liver abscesses (arrow), with normal appearances to the main left and right intrahepatic ducts and common bile duct.
He continued on rotational antibiotics (oral ciprofloxacin/co-amoxiclav), with serial MRCP/MRI liver confirming improvement in the abscesses. He was readmitted 10 months later with worsening jaundice, abdominal pain, fevers, pruritus and weight loss. Repeat MRI liver as an inpatient confirmed resolution of previous abscesses and the absence of both IHD and CBD dilation. MDR3 canalicular phospholipid export pump genetic mutations (known to predispose to recurrent cholestasis) were also excluded by blood screening. Liver biopsy was performed, confirming features consistent with chronic cholangiopathy (figure 2).
Figure 2.
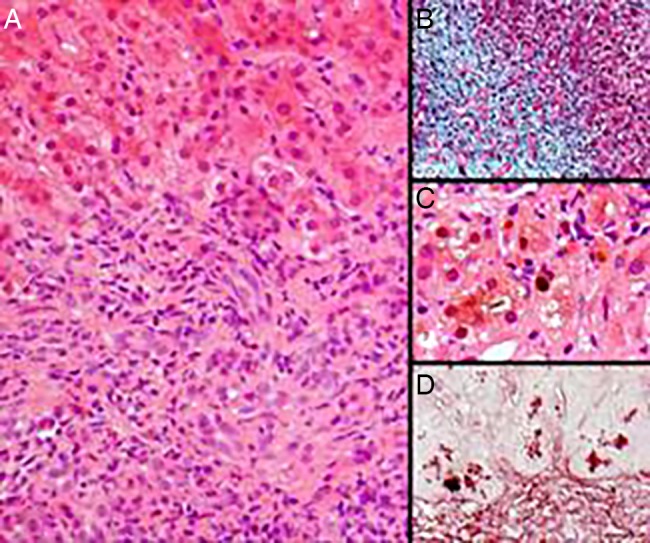
Chronic cholangiopathy in liver biopsy. (A) Portal expansion by fibrous tissue with mixed inflammatory cell infiltrates. (B) Trichrome staining showing portal fibrosis with focal ductular proliferation. (C) Hepatocellular and canalicular cholestasis. (D) Orcein staining showing periportal granules of copper-associated protein.
Endoscopic retrograde cholangiopancreatography (ERCP, figure 3) showed multiple irregular strictures, beading and filling defects exclusively in the right hepatic duct, and sphincterotomy was performed. Multiple black biliary casts and sludge were retrieved from the right main hepatic duct by balloon trawl and bile cultures grew amoxicillin sensitive Enterococcus faecalis. None of these changes, including filling defects, had been demonstrated on previous MRCP, even in retrospect. In view of prolonged cholestasis, ursodeoxycholic acid was commenced and cholestyramine and chlorphenamine for pruritus. Temporary clinical and biochemical improvement followed, and the patient re-presented 6 weeks later with jaundice, fevers, pain and pruritus. ERCP was repeated with identical findings; a plastic stent was inserted into the right hepatic duct and removed 3 months later, with cholangiography confirming adequate cast clearance again.
Figure 3.
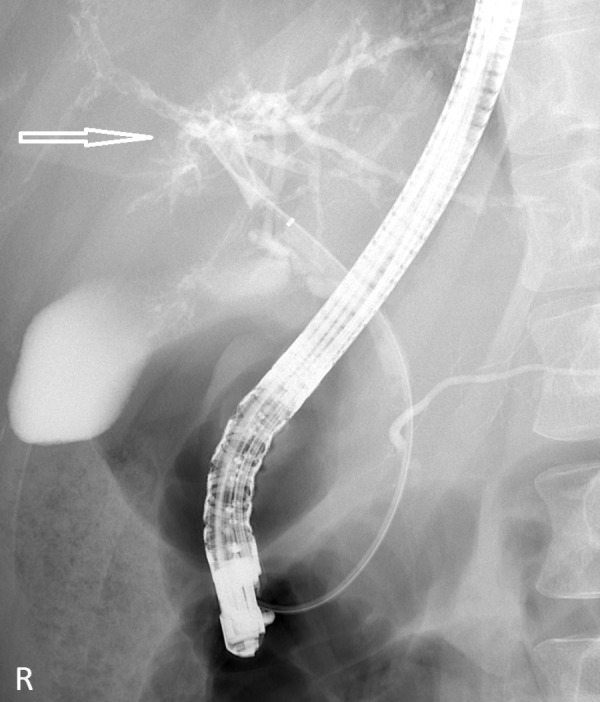
First endoscopic retrograde cholangiopancreatography confirming multiple irregular strictures, beading and filling defects exclusively in the right hepatic duct (arrow).
The above cycle continued to repeat despite rotational antibiotics and adequate intervention at numerous ERCPs (nine ERCPs over 24 months including prolonged nasobiliary flushing with normal saline and direct ‘Spyglass’ enteroscopy). Cholangiograms have confirmed progression, and now both the right and left hepatic ducts are involved with stricturing and casts. Repeat liver biopsy has confirmed progression of portal tract fibrosis without progression to cirrhosis. Colonoscopy to exclude UC and associated PSC was unremarkable.
Outcome and follow-up
Over time he has become antibiotic dependent (repeated 4-weekly courses of co-amoxiclav and then ciprofloxacin) and has multiple fat-soluble vitamin deficiencies. The most recent ERCP has not shown further solid material, and the combination of antibiotics and adequate therapeutic ERCP has stabilised the condition at present (table 1). He has regular outpatient follow-up to ensure progression to cirrhosis or complications of portal hypertension have not developed. He is aware he is likely to require liver transplantation in due course.
By exclusion of other causes and the similarity to other cases of SSC-CI reported elsewhere, the hypoxic/ischaemic critical illness appears to be the cause of the patient’s cholangiopathy.1 2
Discussion
Thirty per cent of patients in medical intensive care will develop abnormal liver function tests, most within the first 48 h.4 There are two common patterns of liver injury in critically ill patients without pre-existing liver disease: jaundice with cholestasis which will develop in about 20% and hypoxic liver injury (HLI, also known as ischaemic hepatitis or ‘shock’ liver), which will develop in about 10%.4
HLI usually occurs in an acute setting of cardiac, circulatory or respiratory failure, with a transient increase in serum aminotransferase levels to >20 times the upper limit of normal, and the diagnosis is made after exclusion of other potential causes for an acute hepatitis (eg, viral, drug-related).4
Jaundice with cholestasis of critical illness is characterised by functional changes affecting hepatocytes, and usually occurs in the absence of mechanical biliary tree obstruction. Serum bilirubin, alkaline phosphatase and γ-glutamyl transpeptidase are more significantly elevated than serum aminotransferase.4 Hepatocellular injury is presumed to lead to decreased bilirubin uptake by the hepatocyte, decreased intrahepatic processing, decreased canalicular transport and decreased conjugated bilirubin clearance. It is usually precipitated by sepsis or septic shock, and other causes include haemodynamic instability, renal insufficiency, hepatotoxic drugs, multiple blood transfusions, increased positive end-expiratory pressure levels and total parenteral nutrition.4 The majority of cases display reversible functional damage; however, in a small proportion there is progression to SSC.1 4
SSC-CI remains a rare progression of jaundice and cholestasis in critically ill patients with fewer than 100 reported cases worldwide, mostly from Germany, and at least five with critical illness secondary to H1N1 influenza.2 3 5 6 There is no association with underlying hepatobiliary disease. The cholestatic liver dysfunction persists despite resolution of the primary pathology causing the critical illness. Individuals with genetic variants to the MDR3 canalicular phospholipid export pump or other hepatobiliary disease are likely to have a more severe disease course with faster progression to cirrhosis.1
Common to most reported cases are the following:
Severe life-threatening illness requiring prolonged intensive care treatment
Respiratory insufficiency/acute respiratory distress syndrome requiring significant ventilatory support
Prolonged vasopressor requirement
Numerous biliary casts in the IHDs at ERCP
Persistent cholangitis, usually secondary to Enterococcus faecium or E faecalis
Recurrence of cholangitis symptoms despite adequate ERCP intervention, with ongoing progressive biliary tree destruction
The pathogenesis is thought to be secondary to biliary ischaemic injury.1 2 Arterial hypoperfusion to the peribiliary vascular plexus (hepatic arterial branches) causes injury to the bile duct epithelium and predisposes to biliary cast formation and biliary stricturing. The IHDs are most predisposed to injury because they exclusively receive blood supply only from the peribiliary vascular plexus, and critically ill patients are prone to this because both vasopressor administration and lung protective mechanical ventilation (with high positive end-expiratory pressure and a low tidal volume) compromise hepatosplanchnic haemodynamics. Biliary cast formation and stricturing thereafter predispose to refractory cholangitis.
Early signs of SSC-CI include conjugated hyperbilirubinaemia and rapidly rising alkaline phosphatase. ERCP is the gold standard diagnostic investigation for SSC-CI and typically shows sclerosis, multiple consecutive strictures, intrahepatic biliary casts and biliary sludge. Liver histology confirms cholangiopathy2 and CT and MRCP are frequently uninformative.6
Complications include the disruption of immune responses leading to attenuated protection from bacterial infection, altered metabolism of lipid and glucose and acute tubular necrosis in the short term.1 Long-term complications are chronic cholangitis, fat-soluble vitamin deficiencies and liver cirrhosis.1 Pruritus related to cholestasis should be treated conventionally.
Therapeutic ERCP with duct clearance and biliary stenting or nasobiliary drain (with saline flushing) reduces the burden of intrahepatic casts and biliary sludge transiently. In conjunction with antibiotics, this controls cholangitis and ultimately bridges to liver transplant assessment. Data from a single series suggest that 30% will require liver transplantation within 2 years of diagnosis.2 Consideration for liver transplantation and referral to a transplant centre should be timely, in accordance with local policies (generally when complications of cirrhosis develop, or there is persistent itching not responding to medical therapy, or there is severe recurrent cholangitis) and ideally before the development of resistant bacteria.
To the best of our knowledge, this is the first reported case of SSC-CI in the UK. This syndrome is not recognised in the UK despite several international reports, and therefore we highlight the presentation, progression and management. SSC-CI should always be considered when there is protracted cholestasis and cholangitis after critical illness, specifically in the absence of an alternative diagnosis.
Learning points.
Cholestatic dysfunction during critical illness can rarely progress to secondary sclerosing cholangitis (SSC-CI), presenting as persistent jaundice, right-sided abdominal pain, pruritus and cholangitis.
SSC-CI can be diagnosed by endoscopic retrograde cholangiopancreatography (ERCP) and liver biopsy. The presence of numerous intrahepatic biliary casts in an individual with ongoing cholestasis following a critical illness suggests SSC-CI. MRCP and CT are uninformative.
Rotational antibiotics and therapeutic ERCP (with sphincterotomy, duct clearance and biliary stenting or nasobiliary drainage with saline flushing) are only of limited efficacy in patients with SSC-CI and serve as a bridge to definitive treatment.
SSC-CI is an indication for liver transplant assessment when there is incipient cirrhosis.
Footnotes
Contributors: KVP and SZ drafted the case and obtained consent from the patient to publish. MW critically revised the manuscript and is the gastroenterologist responsible for the patient. FC reviewed all histology and provided the slides for the report.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ruemmele P, Hofstaedter F, Gelbmann CM. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol 2009;6:287–95 [DOI] [PubMed] [Google Scholar]
- 2.Gelbmann CM, Rümmele P, Wimmer M, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol 2007;102:1221–9 [DOI] [PubMed] [Google Scholar]
- 3.Kirchner GI, Scherer MN, Obed A, et al. Outcome of patients with ischemic-like cholangiopathy with secondary sclerosing cholangitis after liver transplantation. Scand J Gastroenterol 2011;46:471–8 [DOI] [PubMed] [Google Scholar]
- 4.Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care 2013;19:128–32 [DOI] [PubMed] [Google Scholar]
- 5.Weig T, Schubert MI, Gruener N, et al. Abdominal obesity and prolonged prone positioning increase risk of developing sclerosing cholangitis in critically ill patients with influenza A-associated ARDS. Eur J Med Res 2012;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon ON, Cho SH, Park CK, et al. Biliary cast formation with sclerosing cholangitis in critically ill patient: case report and literature review. Korean J Radiol 2012;13:358–62 [DOI] [PMC free article] [PubMed] [Google Scholar]


