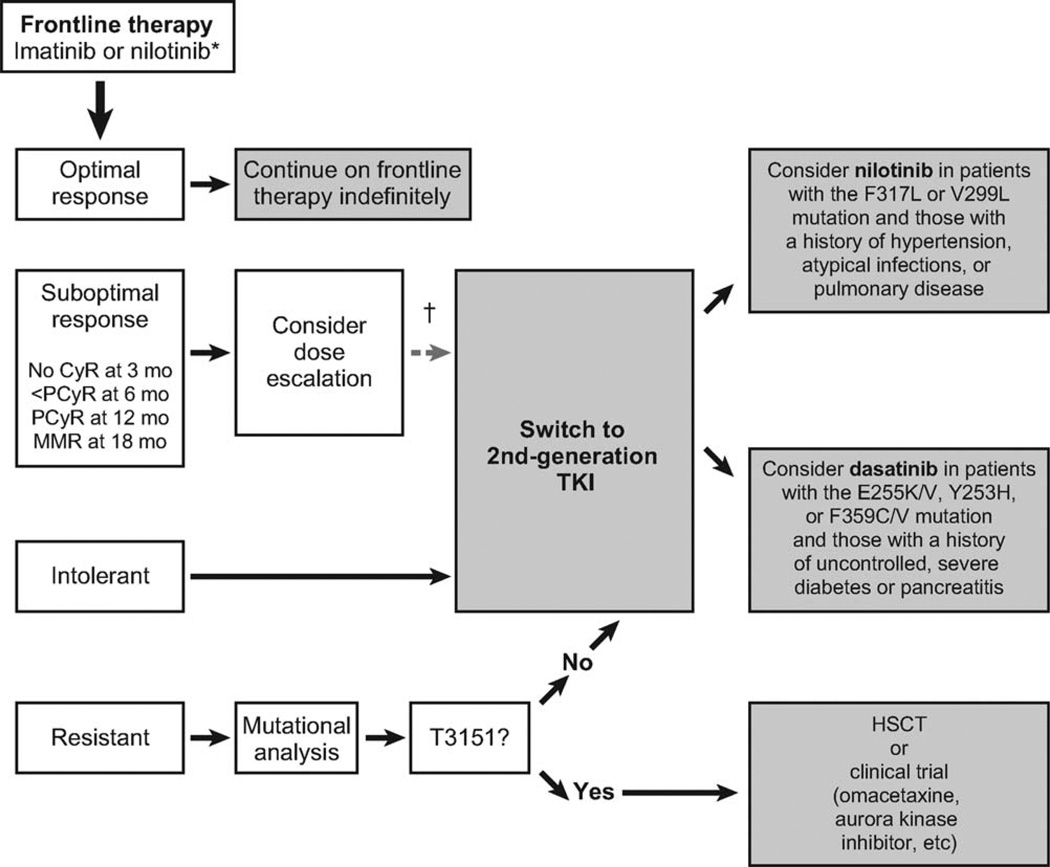
Figure 2.
This chart illustrates the suggested treatment algorithm for patients with newly diagnosed chronic myeloid leukemia (CML) in chronic phase. An asterisk indicates that imatinib and nilotinib are the only approved tyrosine kinase inhibitors (TKIs) for the frontline treatment of CML. Nilotinib recently was approved by the US Food and Drug Administration after it demonstrated superior response rates and a significant improvement in the time to progression compared with imatinib36; those improvements persisted at a median follow-up >18 months in an international, phase 3, randomized trial.40,41 Dasatinib also demonstrated superior response rates compared with imatinib in a phase 3 randomized trial and is under review for regulatory approval in several countries.29 Bosutinib is being evaluated in a randomized trial of patients with newly diagnosed CML, and those results are expected in 2010. The dagger indicates that randomized clinical trials currently are underway to examine the value of an early switch to second-generation TKIs in patients who have a suboptimal response to imatinib. CyR indicates cytogenetic response; PCyR, partial cytogenetic response; MMR, major molecular response; F317L, phenylalanine to leucine mutation at codon 317; V299L, valine to leucine mutation at codon 299; E255K/V, glutamic acid to lysine or valine mutation at codon 255; Y253H, tyrosine to histidine mutation at codon 253; F359C/V, phenylalanine to cysteine or valine mutation at codon 359; HSCT, hematopoietic stem cell transplantation.