Figure 6. Domains of telomeric dsDNA binding proteins.
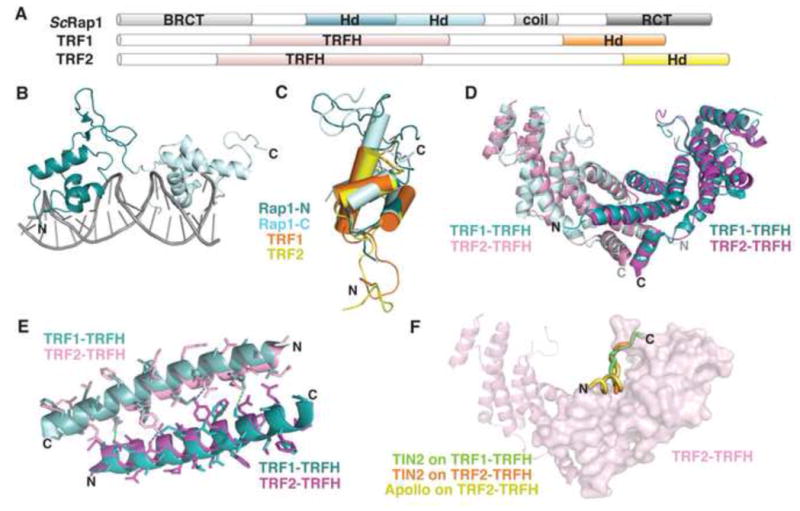
(A) Domain topology of TRF1, TRF2, and ScRap1. (B) ScRap1 binds to double-stranded telomeric DNA with two homeodomains (N-terminal, teal; C-terminal, light blue; PDB: 1IGN). (C) Overlay of the homeodomains from ScRap1 (ScRap1-N, teal; ScRap1-C, light blue; PDB: 1IGN), TRF1-DBD (orange; PDB: 1ITY), and TRF2-DBD (yellow; PDB: 1VF9). (D) Superposition of the TRF1-TRFH homodimer (aqua and light blue; PDB: 1H6O) with the TRF2-TRFH homodimer (pink and magenta; PDB: 1H6P; rmsd =1.23Å). (E) Superposition of the α1 helices that comprise part of the homodimerization interface. TRF1, teal and light blue; TRF2, pink and magenta. Dashes indicate hydrogen bonding between monomers (TRF1, green; TRF2, blue). (F) Superposition of three peptide ligands on the structure of the TRF2-TRFH homodimer. TIN2 peptide bound to TRF1, green (PDB: 3BQO); TIN2 peptide bound to TRF2, orange (PDB: 3BU8); Apollo peptide bound to TRF2, yellow (PDB: 3BUA). For simplicity, only one peptide binding site is shown on the surface of one monomer in the homodimer.
