Abstract
Background
Schizophrenia has a neurodevelopmental component to its origin, and may share overlapping pathogenic mechanisms with childhood neurodevelopmental disorders (ND). Yet longitudinal studies of psychotic outcomes among individuals with ND are limited. We report a population-based prospective study of six common childhood ND, subsequent neurocognitive performance and the risk of psychotic experiences (PEs) in early adolescence.
Methods
PEs were assessed by semi-structured interviews at age 13 years. IQ and working memory were measured between ages 9 and 11 years. The presence of six neurodevelopmental disorders (autism spectrum, dyslexia, dyspraxia, dysgraphia, dysorthographia, dyscalculia) was determined from parent-completed questionnaire at age 9 years. Linear regression calculated mean difference in cognitive scores between those with and without ND. The association between ND and PEs was expressed as odds ratio (OR); effects of cognitive deficits were examined. Potential confounders included age, gender, father’s social class, ethnicity and maternal education.
Results
Out of 8,220 children, 487 (5.9%) were reported to have ND at age 9 years. Children with, compared with those without ND performed worse on all cognitive measures; adjusted mean difference in total IQ 6.84 (95% CI 5.00- 8.69). The association between total IQ and ND was linear (p<0.0001). The risk of PEs was higher in those with, compared with those without ND; adjusted OR for definite PEs 1.76 (95% CI 1.11- 2.79). IQ (but not working memory) deficit partly explained this association.
Conclusion
Higher risk of PEs in early adolescence among individuals with childhood ND is consistent with the neurodevelopmental hypothesis of schizophrenia.
Keywords: Neurodevelopmental Disorder, Dyslexia, Dyspraxia, Autism, Autism Spectrum Disorder, Dyscalculia, Dysgraphia, Dysorthographia, Childhood, Psychotic Experiences, Psychotic Symptoms, IQ, Working Memory, Neurodevelopment, Neurocognitive Performance, Schizophrenia, Psychotic Disorder, Mediation Analysis, Risk, Birth Cohort Study, ALSPAC
Introduction
The neurodevelopmental hypothesis of schizophrenia posits abnormal brain development as a cause of this illness (Murray & Lewis 1987; Weinberger 1987). Empirical support for this hypothesis comes from population-based longitudinal studies demonstrating an association between subtle alterations in motor, cognitive, language and social development in the early life and the risk of adult schizophrenia (Cannon et al. 2002; Cannon et al. 2000; Crow et al. 1995; Jones et al. 1994). Common neurodevelopmental disorders of childhood such as autism, dyslexia, dyspraxia share many similarities with schizophrenia (Bassett et al. 2010; Owen et al. 2011), which typically manifests itself in young adulthood. These conditions are more common in men, associated with cognitive deficits, and neurological soft signs (Owen et al. 2011). Genetic studies suggest an overlap of risk between schizophrenia, autism and other neurodevelopmental conditions such as attention deficit hyperactivity disorder (ADHD) (Bassett et al. 2010; Kirov et al. 2009; Williams et al. 2010). Family history of schizophrenia is associated with risk of autism (Sullivan et al. 2012). It has been suggested that childhood autism and adult schizophrenia share overlapping pathogenic mechanisms arising from disruptions in brain development (Owen et al. 2011).
Although there is substantial inter-individual variation within common childhood neurodevelopmental disorders, there is also a great deal of overlap in their clinical presentation and aetiology (Richardson & Ross 2000). Specific language impairments in autism and dyslexia may be underpinned by the same genetic aberration (Vernes et al. 2008). Evidence of neurocognitive deficits such as low IQ or processing speed in autism, dyspraxia and dyscalculia suggest that there is some degree of neurodevelopmental aberrations in these conditions (Butterworth & Reigosa 2007; Matson & Shoemaker 2009; Scalais et al. 2005). Therefore, an increased risk of psychotic outcomes in the future among individuals with these disorders in childhood will be consistent with the neurodevelopmental view of schizophrenia. However, longitudinal studies of schizophrenia among individuals with childhood neurodevelopmental disorder are limited (Hutton et al. 2008).
It has been suggested that childhood psychotic experiences (PEs) may be important antecedents of adult schizophrenia. These are associated with risk of psychotic illness in adult life as well as a number of risk factors for schizophrenia (Horwood et al. 2008; Kelleher & Cannon 2011; Polanczyk et al. 2010; Poulton et al. 2000; Zammit et al. 2013). Recently, two studies have reported an increased risk of PEs in early adolescence for autistic traits (speech problem, social problem, rituals) (Bevan Jones et al. 2012), or a diagnosis of autism spectrum disorder (ASD) in childhood (Sullivan et al. 2013). Similar to the current study, these were based on data from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort. However, they did not include any other neurodevelopmental disorder as exposure.
Identification of a linear relationship between IQ deficit in the premorbid period and future risk of schizophrenia is a key piece of evidence underpinning a developmental aspect to the disorder (Khandaker et al. 2011). IQ deficit is present in different stages of schizophrenia and is one of the most important predictors of functional outcome (Gold et al. 2002). Population-based studies have also reported cognitive deficits in childhood among individuals reporting PEs later in childhood or adolescence (Horwood et al. 2008; Niarchou et al. 2013; Polanczyk et al. 2010). Therefore, prospective studies of the effects of IQ and related cognitive factors on the association between childhood neurodevelopmental disorder and later psychotic outcome may help to elucidate the developmental pathways to psychosis.
Using data from the population-based Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, we report associations between six common childhood neurodevelopmental disorders (dyslexia, dyspraxia, dysgraphia, dysorthographia, dyscalculia, and ASD) up to age 9 years, neurocognitive performance assessed as IQ, short term memory, working memory between ages 9 and 11 years, and the risk of PEs at age 13 years. We predicted that neurodevelopmental disorders would increase the risk of PEs, and intermediary neurocognitive deficits would explain this association.
Methods
Description of cohort
The ALSPAC birth cohort is based on all pregnant women resident in the county of Avon, a geographically defined region in the southwest of England, with expected dates of delivery between April 1991 and December 1992 (www.alspac.bris.ac.uk). The initial ALSPAC cohort consisted of 14,062 live births and 13,988 infants still alive at 12 months (Boyd et al. 2013; Fraser et al. 2013). Avon included both urban and rural areas, and the population was broadly representative of all children in the UK. The parents completed regular postal questionnaires about all aspects of their child’s health and development since birth. Since the age of 7 years the children attended an annual assessment clinic during which they participated in a range of face-to-face interviews and physical tests. This study is based on 8,220 individuals with data on neurodevelopmental disorders at age 9 years.
Ethical approval for the study was obtained from ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Assessment of neurodevelopmental disorders
The ALSPAC parents completed a questionnaire when the study child was on average 9 years old. A variety of questions were asked about health and development of the child including a specific item on neurodevelopmental disorders. In that item, a lead in, “Have you ever been told that your child has:” was followed by a list of six specific disorders: Dyslexia (developmental reading disorder); Dyspraxia (coordination disorder); Dysgraphia (difficulties in writing); Dysorthographia (difficulties in spelling); Dyscalculia (inability to learn or comprehend arithmetic); Autism, Asperger’s syndrome or Autistic Spectrum Disorder. For each disorder the parent ticked ‘yes’ or ‘no’; if ‘yes’, the child’s age at diagnosis. Using this data we created a single binary variable, ‘any neurodevelopmental disorder’ (ND), which was used as the primary exposure.
Assessment of neurocognitive performance
IQ and short-term memory (average age 9 years)
IQ was measured by the Wechsler Intelligence Scale for Children (WISC III, 3rd UK edition) (Wechsler et al. 1992). A shortened version of the test was applied by trained psychologists, whereby only alternate items were used for all subtests with the exception of the coding subtest which was administered in its standard form. Digit span subtest of WISC III was used as a measure of short-term memory.
Working memory (average age 11 years)
The computerised Counting Span Task was used (Case et al. 1982), which simultaneously tests information processing and storage abilities. A child’s working memory span was calculated automatically by the computer programme. The maximum score a child could achieve was five (i.e. all correct). We used the span score, the main outcome measure for this task.
Assessment of psychotic experiences
Psychotic experiences (PEs) were assessed by the semi-structured Psychosis-like Symptoms interview (PLIKSi) at a mean age of 12.9 years (SD 0.23). The PLIKSi comprised 12 ‘core’ questions derived from the Diagnostic Interview Schedule for Children–IV (DISC–IV) (Shaffer et al. 2000), and the Schedules for Clinical Assessment in Neuropsychiatry version 2.0 (SCAN 2.0) (WHO 1994). It included key symptoms covering the three main domains of positive psychotic symptoms: hallucinations (visual and auditory); delusions (delusions of being spied on, persecution, thoughts being read, reference, control, grandiose ability and other unspecified delusions); and experiences of thought interference (thought broadcasting, insertion and withdrawal). This allowed an observer-based rating for the presence of any psychotic experiences in the last six months (further classed as suspected and definite). We used any PEs as the primary outcome, and definite PEs as a secondary outcome. These groups were compared with rest of the cohort. The PLIKSi has good inter-rater reliability (kappa=0.7), and test-retest reliability (kappa=0.5) (Horwood et al. 2008). The interview and coding procedure have been reported in detail elsewhere (Horwood et al. 2008).
Statistical analysis
Linear regression compared mean scores of IQ and memory tasks between those with and without ND. Mean difference (95% CI) between groups was calculated for each task. Age at the time of testing (in days), gender, father’s social class and ethnicity were included as potential confounders. Logistic regression examined the association between total IQ score and ND. Linearity of association between IQ and ND was examined by including a quadratic term (square of IQ score) within the logistic regression model.
Binary logistic regression calculated odds ratio (OR) for PEs in those with, compared with those without ND. Age at the time of assessment of PEs (in weeks), gender, ethnicity, father’s social class and maternal education were included as potential confounders. Due to limited number of individuals with PEs, multivariable regression was used to calculate ORs separately only for dyslexia. In addition, we used the likelihood-ratio test to examine whether an alternative approach to defining the exposure variable provided a better fit to the data compared with the null, binary coding (any ND vs. none). The alternative coding of the exposure included five discrete categories (No ND; dyslexia only; dyspraxia only; ASD only; multiple ND).
We examined whether the association between any PEs and ND could be explained by deficit in total IQ (Baron & Kenny 1986). First, separate logistic regression models assessed the associations between: (1) exposure (ND) and outcome (PEs); (2) exposure and mediator (total IQ score); (3) mediator and outcome, controlling for exposure. Finally, exposure-outcome, exposure-mediator, mediator-outcome, all three regression lines were fitted simultaneously in a single model using MPlus. We expected, in the final step, the exposure-outcome relationship would be attenuated (partial mediation), or eliminated (complete mediation). In case of partial mediation, the extent to which the ND-PEs estimate was attenuated after inclusion of total IQ (the mediator) was also calculated. This procedure was repeated using working memory as the potential mediator. Mediating effects of IQ and working memory on the association between dyslexia and PEs were also examined.
Sensitivity analyses
In order to examine the association between any neurodevelopmental disorder, IQ and PEs more rigorously, we repeated all analyses after excluding cases of ASD (i.e. any neurodevelopmental disorder included dyslexia, dyspraxia, dyscalculia, dysgraphia, and dysorthographia but not ASD). This is because two previous studies from the ALSPAC cohort have reported an association between autism and later PEs (Bevan Jones et al. 2012; Sullivan et al. 2013).
Results
Frequency of neurodevelopmental disorders at age 9 years and baseline characteristics
Out of total 8,220 children, 487 (5.9%) were reported to have a neurodevelopmental disorder at age 9 years. Out of these, 417 children (5.1%) had only one, whilst 70 (0.9%) had more than one disorder. Dyslexia was the most common (Figure 1); dysgraphia and dysorthographia were reported to be present in the same 24 children. Neurodevelopmental disorders (ND) were more common in boys (Table 1).
Figure 1. Frequency of parent-reported neurodevelopmental disorders at age 9 years in the ALSPAC birth cohort.

Table 1. Baseline characteristics of individuals with and without neurodevelopmental disorders in ALSPAC.
| Group/ characteristics | Neurodevelopmental disorder |
No neurodevelopmental disorder |
|---|---|---|
|
| ||
| Total number | 487 | 7,733 |
|
| ||
| Age at PE, mean (SD) in years | 12.90 (0.26) | 12.87 (0.21) |
|
| ||
| Male (%) | 65.9 | 49.6 |
|
| ||
| British White (%) | 98.9 | 98.3 |
|
| ||
| Father’s Social class (%) | ||
| I | 14.6 | 12.8 |
| II | 42.7 | 35.8 |
| III non manual | 10.3 | 11.8 |
| III manual | 23.3 | 28.6 |
| IV | 6.2 | 8.3 |
| V | 2.9 | 2.5 |
| Armed forces | 0.0 | 0.2 |
|
| ||
| Maternal education (%) | ||
| Secondary school | 9.8 | 14.2 |
| Vocational | 7.0 | 8.7 |
| O level | 36.5 | 35.3 |
| A level | 28.2 | 25.6 |
| Degree | 18.4 | 16.2 |
Neurodevelopmental disorders at age 9 years and neurocognitive performance between ages 9 and 11 years
Compared with children with no neurodevelopmental disorder, children with neurodevelopmental disorder at age 9 years, as a group, performed worse on all measures of IQ, short-term memory and working memory between ages 9 and 11 years (Table 2). The results were very similar after adjusting for a number of potential confounders.
Table 2. Neurocognitive performance in children with and without neurodevelopmental disorders in ALSPAC.
| Cognitive ability and average age of testing | Neurodevelopmental disorder | No neurodevelopmental disorder | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Mean difference (95% CI) | Adjusted mean difference (95% CI)† | |
| Age 9 years | ||||||
| Total IQ | 340 | 98.80 (16.99) | 5919 | 105.36 (16.20) | 6.55 (4.78- 8.33) | 6.84 (5.00- 8.69) |
| Verbal IQ | 345 | 101.99 (17.51) | 5941 | 108.24 (16.54) | 6.25 (4.45- 8.05) | 6.82 (4.95- 8.70) |
| Performance IQ | 343 | 94.78 (18.19) | 5934 | 100.65 (16.79) | 5.87 (4.04- 7.71) | 5.68 (3.75- 7.61) |
| Short-term memory | 333 | 9.01 (2.95) | 5804 | 10.52 (3.06) | 1.51 (1.17- 1.84) | 1.47 (1.12- 1.84) |
| Age 11 years | ||||||
| Working memory | 317 | 3.18 (0.81) | 5685 | 3.44 (0.84) | 0.26 (0.16- 0.36) | 0.28 (0.18- 0.39) |
Adjusted analyses included age at the time of testing, gender, ethnicity, and father’s social class as potential confounders
We also examined total IQ at age 9 years separately in six specific neurodevelopmental disorders (Table 3). Compared with the rest of the cohort, mean total IQ was lower in all disorders except dysgraphia and dysorthographia.
Table 3. Total IQ at age 9 years in children with specific vs. no neurodevelopmental disorder in ALSPAC.
| Specific disorder | Present | Not present | Mean difference (95% CI) | Adjusted mean difference (95% CI)† | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |||
| Dyslexia | 277 | 99.94 (15.62) | 5982 | 105.24 (16.30) | 5.29 (3.33- 7.25) | 5.36 (3.32- 7.41) |
| Dyspraxia | 83 | 97.36 (19.86) | 6177 | 105.10 (16.25) | 7.74 (4.20- 11.27) | 8.62 (4.98- 12.28) |
| Autism spectrum | 48 | 98.62 (18.02) | 6212 | 105.05 (16.30) | 6.42 (1.17- 11.67) | 7.55 (2.80- 12.30) |
| Dyscalculia | 27 | 103.25 (14.47) | 6233 | 105.06 (16.33) | 1.74 (−4.42- 7.92) | 2.50 (−4.11- 9.13) |
| Dysgraphia and dysorthographia‡ | 21 | 106.19 (13.08) | 6239 | 104.99 (16.33) | −1.19 (−8.19- 5.80) | −0.70 (−8.24- 6.83) |
Adjusted analyses included age at the time of testing, gender, ethnicity, and father’s social class as potential confounders.
Dysgraphia and dysorthographia both were reported to be present in these 21 children.
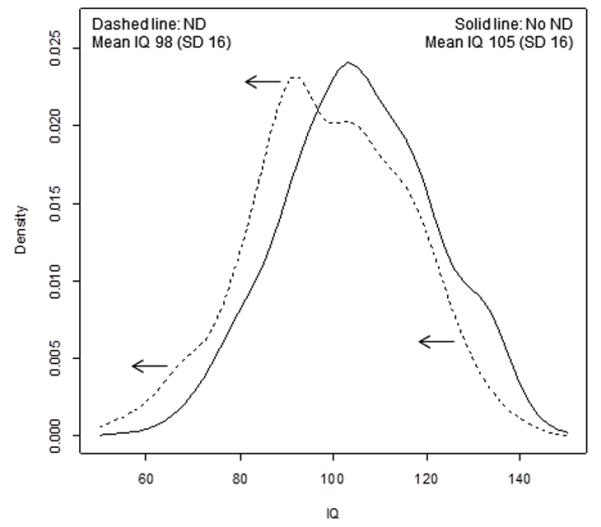
Distribution of IQ scores in children with and without neurodevelopmental disorders
There was a linear association between IQ and ND, consistent with the left-shift of entire distribution of IQ scores in ND; p<0.0001 (Figure 2). The quadratic term (square of IQ) within the logistic regression model of IQ and ND was not significant (p= 0.22). Similar patterns were observed for individual disorders, dyslexia and ASD.
Figure 2. Distribution of total IQ scores in children with and without neurodevelopmental disorders (ND) at age 9 years.
Neurodevelopmental disorders at age 9 years and risk of psychotic experiences at age 13 years
Data on both ND at age 9 years and PEs at age 13 years were available for 5,830 individuals; out of these 313 (5.4%) had ND. In the group with no ND, 711 (12.9%) developed PEs, of which 285 (5.2%) were definite PEs. However, in the group with ND, 58 (18.5%) developed PEs, of which 26 (8.3%) were definite PEs. Similarly, proportions of PEs were also higher in the group with dyslexia, 17.7% any PEs, 8.3% definite PEs.
The risk of PEs was significantly higher among individuals with, compared with those without any neurodevelopmental disorder (Table 4). Evidence of these effects remained after adjusting for age at the time of assessment of PEs, gender, father’s social class, ethnicity and maternal education. Similar increase in risk was also observed for dyslexia and ASD. The likelihood-ratio test for the alternative categorical vs. binary measure of ND was not significant (Chi-squared statistic 0.432; df=4; p=0.97). This provides further evidence that the risk of PEs was not significantly different across discrete categories of ND.
Table 4. Risk of psychotic experiences (PEs) at age 13 years among individuals with neurodevelopmental disorders (ND) at age 9 years.
| ND and adjustment for confounding | N | Odds ratio (95% CI) | |
|---|---|---|---|
| Any PEs | Definite PEs | ||
| Any ND | |||
| Unadjusted | 5830 | 1.53 (1.14- 2.06) | 1.66 (1.10- 2.53) |
| Age and sex | 5830 | 1.55 (1.15- 2.08) | 1.70 (1.12- 2.60) |
| Age, sex, social class, ethnicity, maternal education | 5019 | 1.52 (1.10- 2.12) | 1.76 (1.11- 2.79) |
| Dyslexia | |||
| Unadjusted | 5830 | 1.44 (1.04-2.01) | 1.64 (1.03-2.61) |
| Age and sex | 5830 | 1.44 (1.03- 2.01) | 1.66 (1.05- 2.64) |
| Age, sex, social class, ethnicity, maternal education | 5019 | 1.45 (1.00- 2.09) | 1.90 (1.16- 3.11) |
Effects of neurocognitive deficits on the association between neurodevelopmental disorders and psychotic experiences
In bivariate logistic regression, there was a significant association between ND at age 9 years and risk of PEs at age 13 years (effect estimate 0.234, SE 0.085, p= 0.006). Separate regressions showed significant associations between ND and IQ, IQ and PEs after controlling for ND. Finally, ND-PEs, ND-IQ, IQ-PEs, all three regression lines were fitted simultaneously in a single model using MPlus. In this step, the association between ND and PEs was attenuated but still remained significant (effect estimate 0.195, SE 0.085, p= 0.02). The magnitude of attenuation was 17% (95% CI 10- 25%), suggesting partial mediation of the ND-PEs association by IQ. However, there was no evidence of any effect of working memory scores on the association between ND and PEs. Similarly, the dyslexia-PEs association was attenuated by 16% (95% CI 10- 24%) after including IQ as the potential mediator; working memory did not affect this association.
Sensitivity analyses
The associations between ND, neurocognitive performance and risk of PEs remained virtually unchanged after excluding cases of ASD (N=96) (see online supplementary material).
Discussion
Our findings demonstrate that children with common neurodevelopmental disorders (ASD, dyslexia, dyspraxia, dysgraphia, dysorthographia and dyscalculia), as a group, show significant deficits in a range of neurocognitive domains. We also found that nearly a fifth of these children developed psychotic experiences at the end of follow up. The group with ND at age 9 years, compared with those without had nearly two-fold increased risk of developing psychotic experiences at age 13 years (both suspected/definite PEs and definite PEs only). Evidence of this remained after adjusting for a number of potential confounders. The risk of PEs did not differ significantly across discrete categories of ND. There was a linear association consistent with the left-shift of the distribution of IQ scores in ND, which partly explained the association between ND and PEs. Neurocognitive deficit and increased risk of PEs among individuals with ND, both may be markers of underlying aberration in brain development. Thus, these findings are consistent with the neurodevelopmental hypothesis of schizophrenia (Murray & Lewis 1987; Weinberger 1987).
These findings are also consistent with the notion that neurodevelopmental disorders that typically manifest in childhood (such as, autism) or in young adulthood (such as, schizophrenia) share overlapping pathogenic mechanisms linked with perturbation in brain development (Owen et al. 2011). Cross-sectional studies suggest large proportions of individuals with childhood or adult onset schizophrenia meet criteria for ASD (Rapoport et al. 2009; Unenge Hallerback et al. 2012). However, longitudinal studies of the risk of adult schizophrenia among individuals with childhood autism or other neurodevelopmental disorders are scarce. We could identify only one study, which reported that out of 135 individuals with childhood ASD no one had developed schizophrenia on follow up (Hutton et al. 2008). However, this could be related to the small sample size. Two recent studies from the same cohort as ours have reported an increased risk of PEs in early adolescence for autistic traits (speech problem, social problem, rituals) (Bevan Jones et al. 2012), or a diagnosis of ASD in childhood (Sullivan et al. 2013). However, the current study is distinct in a number of ways. The previous studies focused only on autism. In contrast, the current study examined six common neurodevelopmental disorders including ASD (dyslexia, dyspraxia, dysgraphia, dysorthographia, dyscalculia, and ASD). We also included a number of neurocognitive measures such as IQ, short-term memory, working memory as outcomes. This is important, as effects of cognitive deficits on the association between ND and PEs may shed light on developmental pathways to psychotic disorders.
It has been suggested that psychotic experiences in childhood or adolescence may provide a valid paradigm (‘symptomatic high risk approach’) for studying the development of adult psychotic disorders (Kelleher & Cannon 2011; Murray & Jones 2012). This is supported by a number of observations. Prospective birth cohort studies have reported increased risk of psychotic disorders in adult life among individuals reporting psychotic symptoms in childhood (Poulton et al. 2000; Zammit et al. 2013). Childhood psychotic symptoms are familial and heritable (Polanczyk et al. 2010). They are associated with a number of risk factors for schizophrenia, such as low birth weight (Thomas et al. 2009), cognitive deficits in the premorbid period (Horwood et al. 2008; Niarchou et al. 2013), pregnancy and birth complications (Zammit et al. 2009), and childhood atopic disorders (Khandaker et al. 2013 in press). Population-based studies suggest psychotic symptoms in the general population and those observed in psychotic disorders may exist on a continuum (van Os et al. 2009). Finally, neurophysiological studies have reported common underlying mechanisms for psychotic symptoms occurring in healthy individuals and in schizophrenia (Howes et al. 2013).
We found that the ND-PEs association (also dyslexia-PEs association) could be partly explained by deficit in IQ, but not working memory. This is consistent with current evidence which strongly supports an important role for general cognitive ability as measured by IQ in the pathogenesis and prognosis of schizophrenia. Deficit in IQ, which is present from the premorbid period through to adult life is one of the most consistent findings in schizophrenia epidemiology (Aylward et al. 1984; Jones et al. 1994; Khandaker et al. 2011; Rajji et al. 2009). Besides, it has been reported that IQ is a more sensitive and reliable predictor of functional outcome in schizophrenia than measures of specific ability (Gold et al. 2002).
An explanation for not detecting a larger mediating effect of IQ could be the outcome studied. Adolescent participants with PEs in our sample are almost certainly a heterogeneous group. In many cases these symptoms are likely to be part of development, whilst in some they may be more pathological (De Loore et al. 2011; Rubio et al. 2012). Thus, any underlying biological effect (such as mediation by IQ) may have become diluted. Follow-up of these individuals will be helpful to understand the effect of IQ deficit on different trajectories of early-life PEs.
Limitations of this study include the use of parent reported data rather than diagnosis of neurodevelopmental disorders by formal assessments. However, a number of observations suggest that this parent reported data is acceptable. For example, in our sample at age 9 years 1.2% children were reported to have ASD. A recent population based study by Baron-Cohen and colleagues have reported that the prevalence of ASD is 1.5% among 5-9 year old British school children (Baron-Cohen et al. 2009). Similarly, in our sample prevalence of dyslexia was 4.4%. The prevalence of dyslexia among school age children in England has been reported to be 4- 8% (Hulme & Snowling 2009). Finally, marked discrepancy between verbal and performance IQ has been reported to be a feature of developmental dyspraxia (Scalais et al. 2005). This is reflected in our sample; adjusted mean difference in verbal and performance IQ between children with and without dyspraxia were 4.63 (95% CI 0.94- 8.32) and 12.69 (95% CI 8.90- 16.49), respectively. However, it is difficult to be certain about specific diagnoses from parent reported data. Childhood neurodevelopmental disorders are often comorbid with each other, and there is a great deal of overlap in their presentation and aetiology (Richardson & Ross 2000; Vernes et al. 2008). Therefore, we used any neurodevelopmental disorder as the primary exposure, rather than individual disorders.
A third of children with neurodevelopmental disorders (174 out of 487) did not attend the assessment for PEs at age 13 years. If there was an over-representation of individuals with ND who also developed PEs in our sample, this would lead to spurious overestimation of the ND-PEs association. However, there was no reason to believe that this was the case. In our sample, missing data was associated with lower social class and poorer maternal education. Both of these factors are associated with higher risk of PEs, and over all, poorer health outcomes.
In our sample, neurodevelopmental disorders were relatively more common in children of mothers with better education and in those with higher socioeconomic status. This is consistent with previous studies from both the UK and USA reporting an increased prevalence of autism among individuals with higher socioeconomic status (Thomas et al. 2012; Wing 1980). However, it has been suggested that sampling bias and differential access to healthcare may explain the social class effect in autism. A recent population-based study from Sweden that reported an association between lower parental socioeconomic status and ASD in offspring argues that the burden of autism in lower social class groups previously may have been underestimated (Rai et al. 2012). In ALSPAC, both lower socioeconomic status and poor maternal education is associated with the risk of PEs. Thus, any bias arising from potential misclassification of autism-exposed children with lower socioeconomic status as unexposed might have led to underestimation of the true ND-PEs association in our study.
In order to reduce the length of assessment so the children were less likely to tire, IQ was measured using a shortened version of the WISC III. Alternate items (always starting with item number 1 in the standard form) were used for all ten subtests, except the coding subtest which was administered in its full form. This approach has been successfully used in the past in other studies (Finch & Chihldress 1975; Stricker et al. 1968). We do not have any reliability and validity data for this approach. However, IQ data obtained using this method show robust correlations with other concurrent neurocognitive measures, such as working memory, short-term memory, socio-demographic factors such as social class, age, and subsequent IQ at age 15 years measured by the Wechsler abbreviated scale for intelligence (WASI). Together, they indicate that this may be a time efficient yet reliable way of measuring IQ in large epidemiological studies.
To our knowledge, this is one of the first longitudinal studies of common childhood neurodevelopmental disorders, subsequent neurodevelopment, and the risk of psychotic outcomes. Our findings suggest that future studies of neuropsychological outcomes among individuals with ND should also include psychotic experiences. In the future, birth cohort based neuroimaging studies will be useful to elucidate specific neural networks that underlie the associations between childhood neurodevelopmental disorder, cognitive performance and subsequent psychotic symptoms.
Supplementary Material
Acknowledgment
We are grateful to all families who took part in this study, midwives for their help in recruitment, and the whole ALSPAC team, including interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Funding: Supported by a doctoral clinical research training fellowship from the Wellcome Trust to Dr Khandaker (094790/Z/10/Z); Wellcome Trust (095844/Z/11/Z & 088869/Z/09/Z), and NIHR (RP-PG-0606-1335) grants to Professor Jones, that also supports Dr Stochl. The UK Medical Research Council and the Wellcome Trust grant ref 092731 and the University of Bristol provide core support for ALSPAC.
Abbreviations
- ALSPAC
Avon Longitudinal Study of Parents and Children
- IQ
Intelligence Quotient
- PEs
Psychotic Experiences
- ASD
Autistic Spectrum Disorder
- OR
Odds Ratio
- CI
Confidence Interval
- SD
Standard deviation
Footnotes
Declaration of interest: None of the authors has any conflicts of interest to declare. Prof Jones is co-inventor on patent PCT/GB2005/003279 (methods for assessing psychotic disorders), and received research support from GlaxoSmithKline 2006-10. He directs the National Institute for Health Research Collaborations for Leadership in Applied Health Research and Care for Cambridgeshire and Peterborough (CLAHRC-CP) of which this work forms part.
Reference
- Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull. 1984;10:430–59. doi: 10.1093/schbul/10.3.430. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, Brayne C. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194:500–9. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Scherer SW, Brzustowicz LM. Copy number variations in schizophrenia: critical review and new perspectives on concepts of genetics and disease. Am J Psychiatry. 2010;167:899–914. doi: 10.1176/appi.ajp.2009.09071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan Jones R, Thapar A, Lewis G, Zammit S. The association between early autistic traits and psychotic experiences in adolescence. Schizophr Res. 2012;135:164–9. doi: 10.1016/j.schres.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort Profile: The ‘Children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B, Reigosa V. Information processing deficits in dyscalculia. In: Berch D, Mazzocco M, editors. Why is math so hard for some children? The nature and origins of mathematical learning difficulties and disabilities. Paul H Brookes Publishing; Baltimore, USA: 2007. [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–56. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–93. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Case R, Kurland DM, Goldberg J. Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology. 1982;33:386–404. [Google Scholar]
- Crow TJ, Done DJ, Sacker A. Childhood precursors of psychosis as clues to its evolutionary origins. Eur Arch Psychiatry Clin Neurosci. 1995;245:61–9. doi: 10.1007/BF02190732. [DOI] [PubMed] [Google Scholar]
- De Loore E, Gunther N, Drukker M, Feron F, Sabbe B, Deboutte D, van Os J, Myin-Germeys I. Persistence and outcome of auditory hallucinations in adolescence: a longitudinal general population study of 1800 individuals. Schizophr Res. 2011;127:252–6. doi: 10.1016/j.schres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Finch AJ, Chihldress WB. A comparison of WISC selected subtest short forms with MR children. Mental Retardation. 1975;13:20–21. [PubMed] [Google Scholar]
- Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry. 2002;159:1395–402. doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- Horwood J, Salvi G, Thomas K, Duffy L, Gunnell D, Hollis C, Lewis G, Menezes P, Thompson A, Wolke D, Zammit S, Harrison G. IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. Br J Psychiatry. 2008;193:185–91. doi: 10.1192/bjp.bp.108.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Shotbolt P, Bloomfield M, Daalman K, Demjaha A, Diederen KM, Ibrahim K, Kim E, McGuire P, Kahn RS, Sommer IE. Dopaminergic Function in the Psychosis Spectrum: An [18F]-DOPA Imaging Study in Healthy Individuals With Auditory Hallucinations. Schizophr Bull. 2013;39:807–814. doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C, Snowling MJ. Developmental disorders of language, learning and cognition. Wiley-Blackwell; Chichester: 2009. [Google Scholar]
- Hutton J, Goode S, Murphy M, Le Couteur A, Rutter M. New-onset psychiatric disorders in individuals with autism. Autism. 2008;12:373–90. doi: 10.1177/1362361308091650. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41:1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Barnett JH, White IR, Jones PB. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res. 2011;132:220–7. doi: 10.1016/j.schres.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Zammit S, Lewis G, Jones PB. A population-based study of atopic disorders and inflammatory markers in childhood before psychotic experiences in adolescence. Schizophr Res. 2014;152:139–145. doi: 10.1016/j.schres.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Rujescu D, Ingason A, Collier DA, O’Donovan MC, Owen MJ. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr Bull. 2009;35:851–4. doi: 10.1093/schbul/sbp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009;30:1107–14. doi: 10.1016/j.ridd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Murray GK, Jones PB. Psychotic symptoms in young people without psychotic illness: mechanisms and meaning. Br J Psychiatry. 2012;201:4–6. doi: 10.1192/bjp.bp.111.107789. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J. 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niarchou M, Zammit S, Walters J, Lewis G, Owen MJ, van den Bree MB. Defective processing speed predicts non-clinical psychotic experiences in children; longitudinal analyses in a large birth cohort. Am J Psychiatry. 2013;170:550–557. doi: 10.1176/appi.ajp.2012.12060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, O’Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173–5. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, Moffitt TE, Arseneault L, Cannon M, Ambler A, Keefe RS, Houts R, Odgers CL, Caspi A. Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010;67:328–38. doi: 10.1001/archgenpsychiatry.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–8. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Rai D, Lewis G, Lundberg M, Araya R, Svensson A, Dalman C, Carpenter P, Magnusson C. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. J Am Acad Child Adolesc Psychiatry. 2012;51:467–476 e6. doi: 10.1016/j.jaac.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:286–93. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48:10–8. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AJ, Ross MA. Fatty acid metabolism in neurodevelopmental disorder: a new perspective on associations between attention-deficit/hyperactivity disorder, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins Leukot Essent Fatty Acids. 2000;63:1–9. doi: 10.1054/plef.2000.0184. [DOI] [PubMed] [Google Scholar]
- Rubio JM, Sanjuan J, Florez-Salamanca L, Cuesta MJ. Examining the course of hallucinatory experiences in children and adolescents: A systematic review. Schizophr Res. 2012;138:248–254. doi: 10.1016/j.schres.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Scalais E, Nuttin C, Galluzzo A. Developmental dyspraxia. In: Maria BL, editor. Current Management in Child Neurology. BC Decker Inc.; 2005. pp. 240–245. [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Stricker G, Merbaum M, Tangeman P. WAIS short forms, information transmission and approximation of full scale IQ. Journal of Clinical Psychology. 1968;25:170–172. [Google Scholar]
- Sullivan PF, Magnusson C, Reichenberg A, Boman M, Dalman C, Davidson M, Fruchter E, Hultman CM, Lundberg M, Langstrom N, Weiser M, Svensson AC, Lichtenstein P. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry. 2012;69:1099–1103. doi: 10.1001/archgenpsychiatry.2012.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Rai D, Golding J, Zammit S, Steer C. The Association Between Autism Spectrum Disorder and Psychotic Experiences in the Avon Longitudinal Study of Parents and Children (ALSPAC) Birth Cohort. J Am Acad Child Adolesc Psychiatry. 2013;52:806–814. doi: 10.1016/j.jaac.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Thomas K, Harrison G, Zammit S, Lewis G, Horwood J, Heron J, Hollis C, Wolke D, Thompson A, Gunnell D. Association of measures of fetal and childhood growth with non-clinical psychotic symptoms in 12-year-olds: the ALSPAC cohort. Br J Psychiatry. 2009;194:521–6. doi: 10.1192/bjp.bp.108.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Zahorodny W, Peng B, Kim S, Jani N, Halperin W, Brimacombe M. The association of autism diagnosis with socioeconomic status. Autism. 2012;16:201–13. doi: 10.1177/1362361311413397. [DOI] [PubMed] [Google Scholar]
- Unenge Hallerback M, Lugnegard T, Gillberg C. Is autism spectrum disorder common in schizophrenia? Psychiatry Res. 2012;198:12–7. doi: 10.1016/j.psychres.2012.01.016. [DOI] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–95. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH, Monaco AP, Fisher SE. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–45. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, Golombok S, Rust J. Weschler Intelligence Scale for Children (3rd Edition) (WISC-III UK) The Psychological Corporation; 1992. [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- WHO . SCAN: Schedules for Clinical Assessment in Neuropsychiatry Version 2.0. Psychiatric Publishers International/American Psychiatric Press Inc; Geneva, Switzerland: 1994. [Google Scholar]
- Williams NM, Zaharieva I, Martin A, Langley K, Mantripragada K, Fossdal R, Stefansson H, Stefansson K, Magnusson P, Gudmundsson OO, Gustafsson O, Holmans P, Owen MJ, O’Donovan M, Thapar A. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet. 2010;376:1401–8. doi: 10.1016/S0140-6736(10)61109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing L. Childhood autism and social class: a question of selection? Br J Psychiatry. 1980;137:410–7. doi: 10.1192/bjp.137.5.410. [DOI] [PubMed] [Google Scholar]
- Zammit S, Kounali D, Cannon M, David AS, Gunnell D, Heron J, Jones PB, Lewis S, Sullivan S, Wolke D, Lewis G. Psychotic Experiences and Psychotic Disorders at Age 18 in Relation to Psychotic Experiences at Age 12 in a Longitudinal Population-Based Cohort Study. Am J Psychiatry. 2013;170:742–750. doi: 10.1176/appi.ajp.2013.12060768. [DOI] [PubMed] [Google Scholar]
- Zammit S, Odd D, Horwood J, Thompson A, Thomas K, Menezes P, Gunnell D, Hollis C, Wolke D, Lewis G, Harrison G. Investigating whether adverse prenatal and perinatal events are associated with non-clinical psychotic symptoms at age 12 years in the ALSPAC birth cohort. Psychol Med. 2009;39:1457–67. doi: 10.1017/S0033291708005126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


