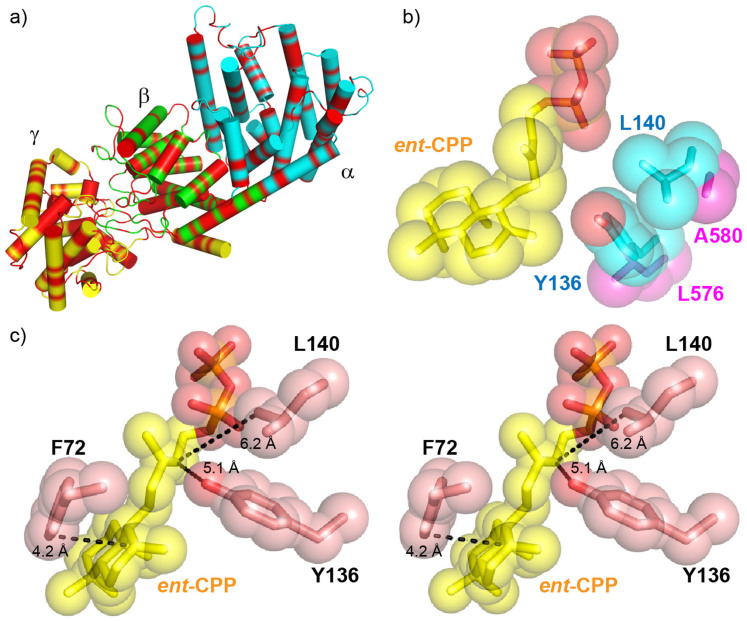
Figure 7. Comparisons between BjKS structure and the tri-functional P. patens CPPS/KS structural model.
(a) Phyre228 homology model of Physcomitrella patens ent-CPPS/ent-KS showing the 45% of residues (in red) that are identical to those in abietadiene synthase. The conserved residues are uniformly dispersed over the α, β and γ domains. (b) ent-CPP (yellow) bound to BjKS (PDB ID 3WBV) superimposed on corresponding P. patens KS residues. The large BjKS residues adjacent ent-CPP are in cyan. The smaller P. patens residues are in magenta and permit water quenching of the kauranyl-16-yl cation. (c) Stereo view of residues that are proposed to stabilize the carbocation intermediates in BjKS (PDB ID 3WBV). Distances between ent-CPP C12 and Y136 O, ent-CPP C12 and L140 Cδ, ent-CPP C8 and F72 C are 5.1 Å, 6.2 Å and 4.2 Å, respectively.