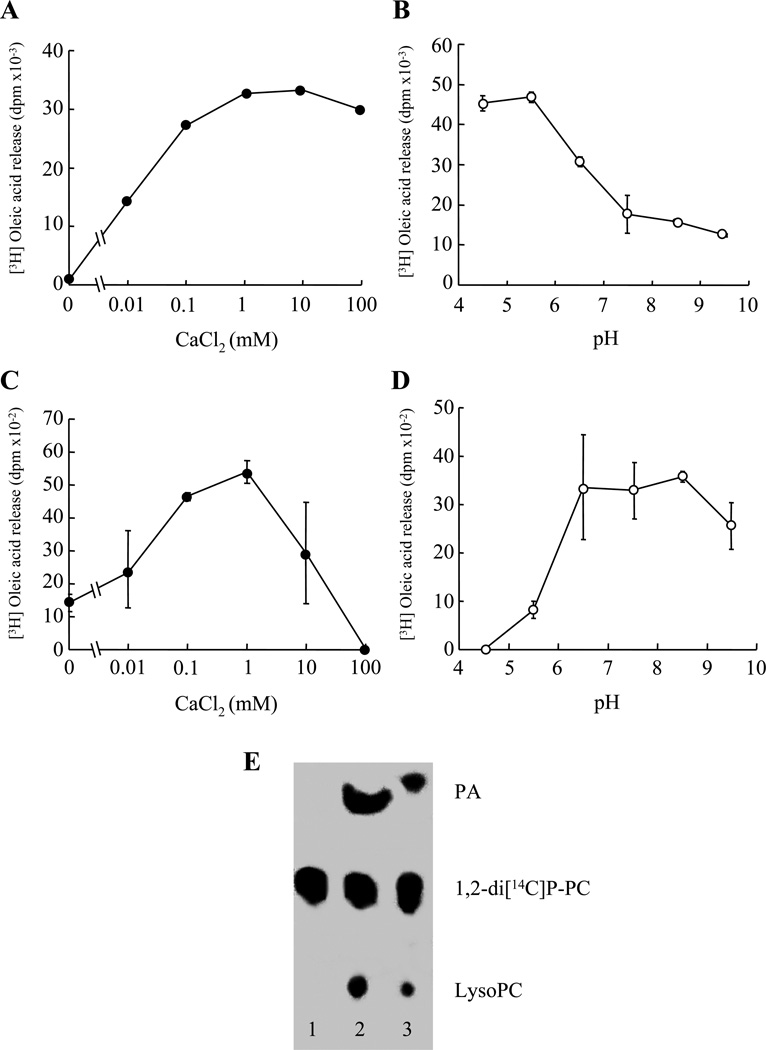
Fig. 2. Enzymatic properties of rsPlaA and rsPlaB produced in E. coli.
(A and C) Calcium dependence of [3H] oleic acid release from radiolabeled bacterial membranes catalyzed by rsPlaA (A) and rsPlaB (C) at pH 5.5 and 8.5, respectively. (B and D) pH dependence of rsPlaA (B) and rsPlaB (D) PLA2 activity in buffers containing 10 mM Ca2+. (E) Thin-layer chromatography and phosphorimager visualization of radiolabeled hydrolysis products from a representative lipolytic assay, carried out under optimized reaction conditions, utilizing double-labeled 1, 2-di[1-14C]palmitoyl L-α-phosphatidylcholine as substrate and either rsPlaA (lane 2) or rsPlaB (lane 3) as enzyme sources (see ‘Materials and Methods’ for details). The substrate (1,2-di[14C]P-PC) and hydrolysis products (palmitic acid, PA; lysophosphatidylcholine, Lyso-PC) are indicated on the right; an enzyme unsupplemented, control reaction is shown in lane 1.