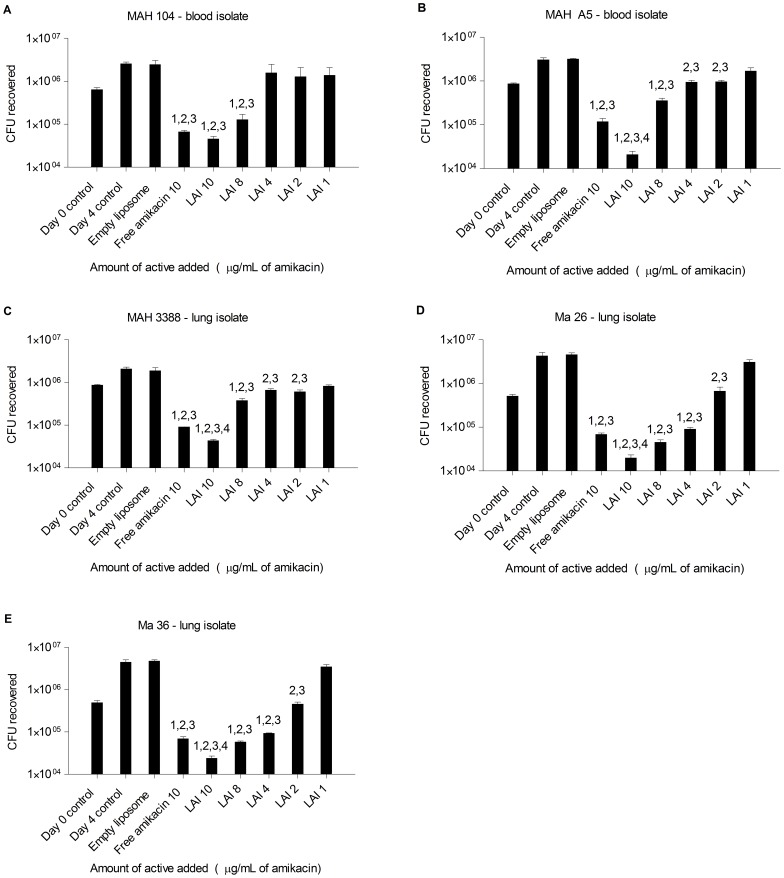
Figure 1. Efficacy of LAI against intracellular mycobacteria in vitro.
LAI, a liposome-encapsulated amikacin compound, was tested against intracellular M. avium subsp. hominissuis (MAH) and M. abscessus (Ma) in vitro. THP-1 human cells (that were first differentiated and adhered with PMA) were infected with a 10∶1 MOI of MAH strains 104 and A5, which are blood isolates (A and B, respectfully), MAH 3388, a lung isolate (C), and Ma strains 26 and 36, which are also lung isolates (D and E, respectfully) for 1 hour. Extracellular bacteria were removed and cells containing internalized bacteria were incubated at 37°C for 24 hours. Wells were either plated for CFU (day 0 control) or treated daily with HBSS (day 4 control), empty liposome, 10 µg/ml amikacin sulfate, or between 10 and 1 µg/ml of LAI for 4 days. Cells were disrupted, diluted, and plated to obtain CFUs of surviving bacteria. Bars represent means of CFU counts and error bars represent standard deviation. Statistical comparisons: 1 = p<0.05 compared to day 0 control; 2 = p<0.05 compared to day 4 control; 3 = p<0.05 compared to empty liposome; 4 = p<0.05 compared to free amikacin.