Figure 1.
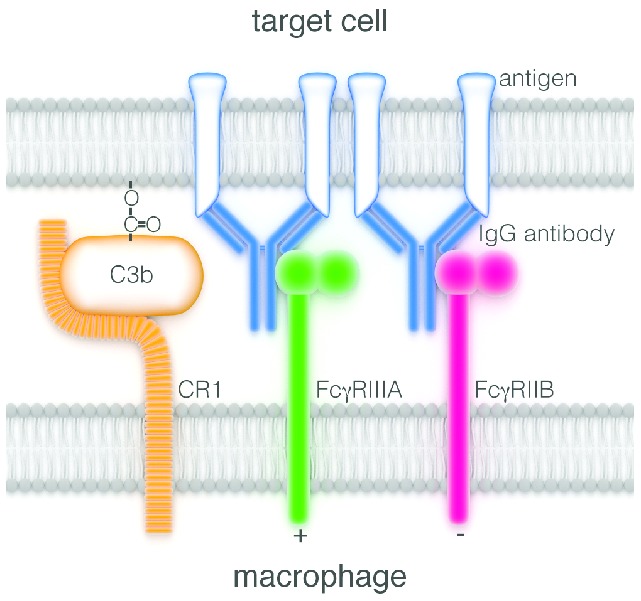
One of the multiple contacts22 forming a synapse between a macrophage and a target cell coated with IgG antibody. Initially antigen molecules have been cross-linked by the bivalent antibody, causing under some circumstances receptor-mediated cytotoxicity. But death is more likely to result from subsequent engagement of antibody Fcγ regions with activating FcγR (here of the class FcγRIIIA) on the macrophage, thus stimulating ADCC activity by the macrophage. This activity is modulated or repressed if inhibitory FcγRIIB receptors are simultaneously engaged. NK cells and granulocytes23 are also capable of killing targets coated with IgG antibody, such encounters being most likely in the bloodstream. NK cells lack FcγRIIB, so any NK cytotoxicity lacks the modulation transmitted by these receptors. The complement system is also activated by the clustered Fcγ regions of coating antibody. Some of the resulting complement products, notably C3b, link covalently to the target-cell surface and might promote opsonization via their affinity for one or other of the macrophage complement receptors (here the receptor CR1 is arbitrarily depicted adjacent to the FcγR). Acting thus complement is a powerful opsonizer for phagocytosis of microorganisms by macrophages and neutrophils, but it has not been consistently shown to assist in attacking antibody-coated tumor cells. It has actually been seen to impede ADCC by NK cells,24 which lack complement receptors. Finally C3b has been reported to have an enigmatic role in promoting antigenic modulation.25
