Abstract
Multiple myeloma is a plasma cell disorder characterized by malignant plasma cell infiltration in the bone marrow, serum and/or urine monoclonal protein and organ damage. The aim of this study was to investigate the impact of chromosome 1 abnormalities in a group of elderly patients (>65 years) with newly diagnosed multiple myeloma enrolled in the GIMEMA-MM-03-05 trial and treated with bortezomib, melphalan and prednisone or bortezomib, melphalan, prednisone and thalidomide followed by bortezomib and thalidomide maintenance. We also evaluated the link between chromosome 1 abnormalities and other clinical, genetic and immunophenotypic features by a multivariate logistic regression model. Interphase fluorescence in situ hybridization on immunomagnetically purified plasma cells and bone marrow multiparameter flow cytometry were employed. A multivariate Cox model showed that chromosome 1 abnormalities, age >75 years and a CD19+/CD117− immunophenotype of bone marrow plasma cells were independent risk factors for overall survival in elderly patients with newly diagnosed multiple myeloma. Moreover, a detrimental effect of thalidomide, even when administered in association with bortezomib, was observed in patients with abnormal chromosome 1 as well as in those with 17p deletion, while the benefit of adding thalidomide to the bortezomib-melphalan-prednisone regimen was noted in patients carrying an aggressive CD19+/CD117− bone marrow plasma cell immunophenotype. This trial was registered at www.clinicaltri-als.gov as #NCT01063179.
Introduction
Multiple myeloma (MM) is a plasma cell disorder characterized by the expansion of clonal plasma cells in the bone marrow (>10%), monoclonal immunoglobulins (Ig) in serum and/or urine and organ damage. Two-thirds of patients with MM are older than 65 years. In Europe, approved therapy for elderly patients or patients not eligible for transplantation is currently based on melphalan (M) and prednisone (P) with thalidomide (T) or bortezomib (V). Recent studies show that lenalidomide, in association with MP or dexamethasone, is a valid alternative.1 Despite the introduction of novel agents in clinical practice, the outcome differs greatly among patients and new prognostic factors are needed to allow patients to be stratified by their risk and, thereby, to be given personalized treatment.1,2
Multiparameter flow cytometry is widely used to characterize bone marrow plasma cells (BMPC) and its impact on defining patients’ prognosis has been investigated by several authors.3–7 Multiparameter flow cytometry is currently the main tool for evaluating minimal residual disease6 during follow-up, while, at diagnosis, cytogenetic abnormalities represent powerful prognostic factors together with the International Staging System (ISS) stage.8–12
Interphase fluorescence in situ hybridization (iFISH) enables identification of the most important genetic aberrations, such as deletion of RB1 [del(13)], P53 [del(17p)], 1p [del(1p)], gain(1q) and IGH translocations.13,14 In a previous study15 two groups of MM patients with different prognoses were identified: the “high-risk group” was characterized by the presence of at least one among del(17p), t(4;14)(p16;q32) and t(14;16)(q32;q23), while the “standard-risk group” was characterized by the absence of any of the aforementioned abnormalities.
Several other chromosomal aberrations have been investigated and gain(1q) has been identified as one of the most recurrent genetic events15 (>50%). Gain(1q) has recently been included in a new cytogenetic classification based on iFISH analysis16: “adverse iFISH”, defined by the presence of one or more of the following aberrations: gain(1q), t(4;14), t(14;16), t(14;20)(p12-p21;q32), and del(17p), and “favorable iFISH”, characterized by the absence of these cytogenetic abnormalities and/or by the presence of hyperdiploidy, t(6;14)(p12-p21;q32) or t(11;14)(q13;q32). Del(1p) is quite a rare event (<10%) and is considered an adverse prognostic factor in young patients.15,16 The relevance of chromosome 1 (chr1) abnormalities has been reported in several studies: Shaughnessy et al. defined a 70-gene high-risk signature, in which 30% of genes mapped to chr1, suggesting the significant poor prognostic impact of gain(1q) and del(1p).17 Moreover, CKS1B overexpression at 1q21 and its involvement in aggressive disease have been described.18 Leone et al. focused on CDKN2C deletion, at 1p32.3, which strongly affects cell-cycle regulation and MM pathogenesis.19 Despite the considerable number of molecular and clinical studies on gain(1q), del(1p) or both20–25, the real role of chr1 abnormalities in MM remains a matter of debate. As far as gain(1q) is concerned, the poor prognostic impact of this aberration has been demonstrated in several series of patients: (i) in newly diagnosed patients, enrolled in the CMG2002 trial, treated with high-dose chemotherapy and autologous stem cell transplantation26; (ii) in patients with recurrent disease, treated with lenalidomide and dexamethasone27; and (iii) in relapsed or refractory patients treated with bortezomib.28
In recent investigations of the efficacy of thalidomide-based regimens in both newly diagnosed and relapsed/refractory MM patients carrying gain(1q21), it was found that thalidomide is not capable of overcoming the adverse influence of gain(1q) on survival.29,30
This retrospective study examines the clinical impact of chr1 aberrations, other common cytogenetic abnormalities and plasma cell immunophenotype in a large series of elderly patients with newly diagnosed MM enrolled in a phase III randomized trial comparing VMP versus VMPT followed by VT maintenance (VMPT-VT).
Methods
Patients
Between 2006 and 2009, 511 elderly (>65 years), untreated MM patients from 61 Italian Hematology Centers were enrolled in a phase III randomized clinical trial comparing VMP versus VMPT-VT31,32. Patients gave written, informed consent before entering the study, which was performed according to the Declaration of Helsinki (Ethics Committee approval number 163/0057512). Bone marrow samples (n=399) were sent to our laboratory for centralized analysis and underwent multiparameter flow cytometry. Of the 399 samples, 376 were purified for routine iFISH analysis. The amount of BMPC allowed evaluation of chr1 abnormalities in 278/376 patients.
Immunophenotype
Four-color multiparameter flow cytometry was performed using CD38 APC, CD138 FITC, CD20 APC, CD45 PerCP, CD19 PerCP-Cy5.5, cytoplasmic κ FITC and λ PE (BD Biosciences), CD117 PE and CD56 PE (Caltag Laboratories) monoclonal antibodies. A FACSCalibur flow cytometer was used for data acquisition, and CELL Quest Pro Software for analysis. An antigen was considered positive when >30% of BMPC expressed it on the cell surface.
Bone marrow plasma cell sorting
BMPC were enriched using anti-CD138-coated magnetic microbeads and an AutoMACS Pro separator (Miltenyi Biotech) following the manufacturer’s instructions, then fixed in Carnoy’s solution. Purity was assessed by multiparameter flow cytometry (plasma cell purity always exceeded 90%).
Interphase fluorescence in situ hybridization
iFISH was performed according to the manufacturer’s instructions. Probes for 1p32, RB1 (on 13q14), and P53 (on 17p13.1) deletions; 1q21 gain and t(11;14)(q13;q32), t(4;14)(p16;q32), t(14;16)(q32;q23) were purchased from Cytocell. Nuclei were analyzed using an Olympus BX41 fluorescent light microscope. Two hundred BMPC nuclei from each sample were scored. The cut-off levels for positive values were the means plus three standard deviations of BMPC from 15 healthy donors, and were adjusted to 15% for IGH translocations and 10% for deletions/gains. Chr 1 patterns were considered positive or negative as shown in Figure 1C,D.
Figure 1.
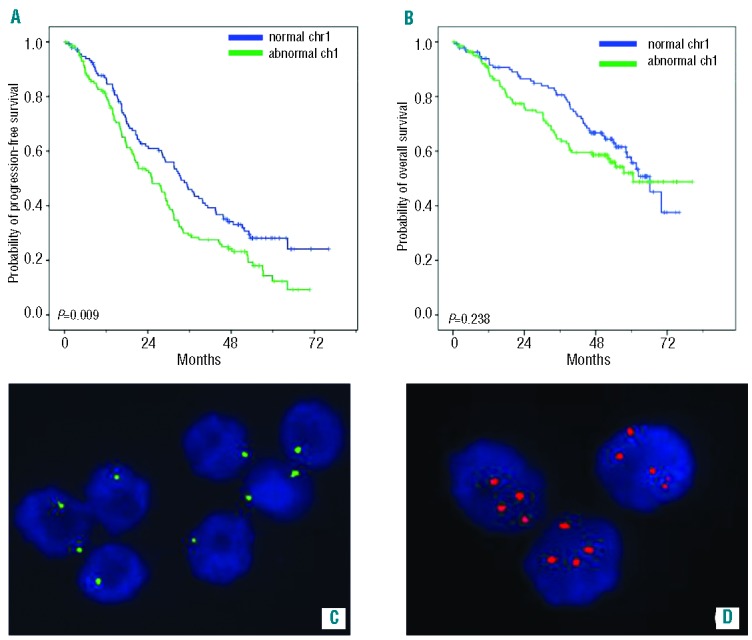
Abnormal chr1: Kaplan Meier curves and iFISH patterns. Clinical outcome of patients carrying abnormal chr1 [del(1p) and/or gain(1q)]: (A) Kaplan-Meier curve for PFS; (B) Kaplan-Meier curve for overall survival; (C) iFISH patterns for del(1p): two green signals in plasma cells with normal 1p and one green signal in plasma cells carrying 1p deletion; (D) iFISH patterns for gain(1q): two red signals in plasma cells with normal 1q and three or more signals in plasma cells carrying 1q gain. (Images captured with a Duet System, BioView Ltd, Israel).
Statistical analysis
The primary end-points were overall survival, defined as the time from study entry to death from any cause, and progression-free survival, defined as the time from study entry until documented disease progression or death from MM. Patients still alive and free of disease progression were censored at the date of last contact.
For univariate analyses, overall and progression-free survival curves were estimated by the Kaplan-Meier method and compared using the log-rank test. Overall and progression-free survival were also analyzed by the Cox proportional hazard model comparing, by the Wald test, chemotherapy (VMPT-VT versus VMP), age at diagnosis (>75 versus ≤75 years), ISS stage (III versus II versus I), abnormal chr1 [del(1p) and/or gain(1q)], del(13), del(17p), t(11;14), [t(4;14) and/or t(14;16)] (any versus none), CD19, CD20, CD45, CD56 and CD117 expression on ≥30% versus <30% of total plasma cells and CD19+/CD117− combination (any versus none). The effect of the same risk factors on overall survival was assessed using a multivariate Cox model. A multivariate binary logistic regression model was used to test age, ISS, iFISH abnormalities (independent variables) as risk factors for the onset of abnormal chr1 (dependent variable).
Patients’ characteristics were tested using the Fisher exact test for categorical variables and the Mann-Whitney test for continuous ones. All reported P-values are two-sided, at the conventional 5% significance level. Data were analyzed as of April 2014 by SPSS 21.0.0 and R 2.15.2 software.
Results
The baseline characteristics of the enrolled patients (n=511) were described in a previous report.31
At the current median follow-up of 54 months from the start of therapy (range, 1 to 80 months), the median progression-free survival is 25 months and the median overall survival has not yet been reached (50.6%).
Chr1 iFISH analysis was performed in 278 patients, based on sample availability. These patients showed the same baseline characteristics as those in whom chr1 abnormalities were not analyzed (Online Supplementary Table SX).
The frequencies of del(13), del(17p), t(11;14), t(4;14), t(14;16) and high-risk chromosomal abnormalities have already been reported by Palumbo et al.31 and are summarized in Table 1 together with the frequency of chr1 abnormalities.
Table 1.
Baseline frequency of chromosomal abnormalities in BMPC detected by iFISH.
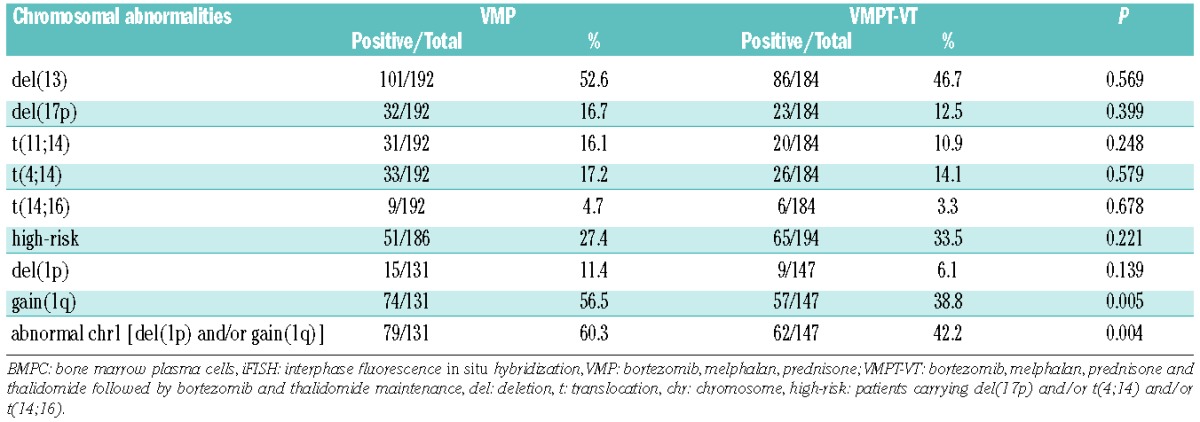
The frequency of chr1 abnormalities was higher in the group treated with VMP than in the group treated with VMPT-VT; this was due to an asymmetric distribution of gain(1q) between the two groups, whereas del(1p) was equally distributed, (Table 1).
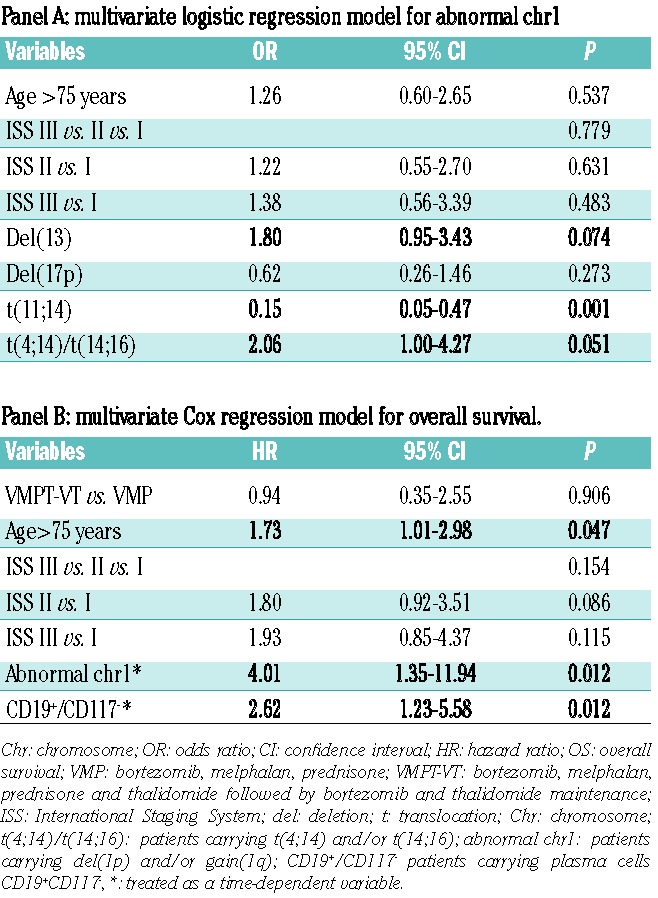
Multivariate logistic regression analysis was used in order to identify protective/risk factors for the presence of an abnormal chr1 and included age, ISS stage and iFISH chromosomal abnormalities [del(13), del(17p), t(11;14) and t(4;14)/t(14;16)] (Table 2, panel A). Del(13) and t(4;14)/t(14;16) were found to be independent risk factors of borderline significance [odds ratio (OR), 1.80; 95% confidence interval (95% CI), 0.95–3.43; P=0.074 and OR, 2.06; 95% CI, 1.00–4.27; P=0.051, respectively) while t(11;14) showed a strong protective role (OR, 0.15; 95% CI, 0.05–0.47; P=0.001). Immunophenotypic features were also tested by logistic regression analysis, but they did not show any significant result (data not shown).
Table 2.
Multivariate regression models.
The associations of chr1 abnormalities with cytogenetic and immunophenotypic features are presented in Online Supplementary Table SZ and Online Supplementary Figure S2).
Figure 1 shows Kaplan-Meier curves for progression-free survival and overall survival according to abnormal chr1 status, highlighting the significant negative impact of such abnormalities on progression-free survival (P=0.009). The presence of chr1 abnormalities appears to have an unusual effect on overall survival, with the impact varying over time, suggesting that this cytogenetic feature should be considered as a time-dependent variable. This hypothesis was confirmed by the Shoenfeld test and, subsequently, Cox analyses were carried out with a time-dependent methodology.
Del(13), del(17p), IGH translocations and high-risk cytogenetics did not significantly affect overall or progression-free survival of enrolled patients (data not shown), except for t(11;14) which displayed a borderline protective role for overall survival [hazard ratio (HR), 0.35; 95% CI, 0.12–1.02; P=0.053].
The immunophenotypic features of BMPC are shown in Online Supplementary Table SY and were equally distributed between the two therapeutic groups. Expression of CD19, CD20, CD45, CD56, CD117 and cytoplasmic k or λ Ig-light-chains did not significantly influence either overall survival or progression-free survival (data not shown). Interestingly, through analysis of several antigen combinations, we identified patients with a CD19+/CD117− immunophenotype as forming a particular risk category for overall survival (HR, 3.51; 95% CI, 1.20–10.31; P=0.022), but not for progression-free survival. This combination was present in 10.3% of all patients and was equally distributed between the VMP and VMPT-VT treatment groups (9.9% versus 10.6%; P=0.871).
Based on univariate Cox analyses, a multivariate Cox regression model for overall survival was tested; the model included chemotherapy, age, ISS stage, chr1 abnormalities and CD19+/CD117− phenotype (Table 2, panel B). Independent predictors for a worse overall survival were age (HR, 1.73; 95% CI, 1.01 to 2.98; P=0.047), abnormal chr1 (HR, 4.01; 95% CI, 1.35 to 11.94; P=0.012) and CD19+/CD117− immunophenotype (HR, 2.62; 95% CI, 1.23 to 5.58; P=0.012).
Differential effect of thalidomide
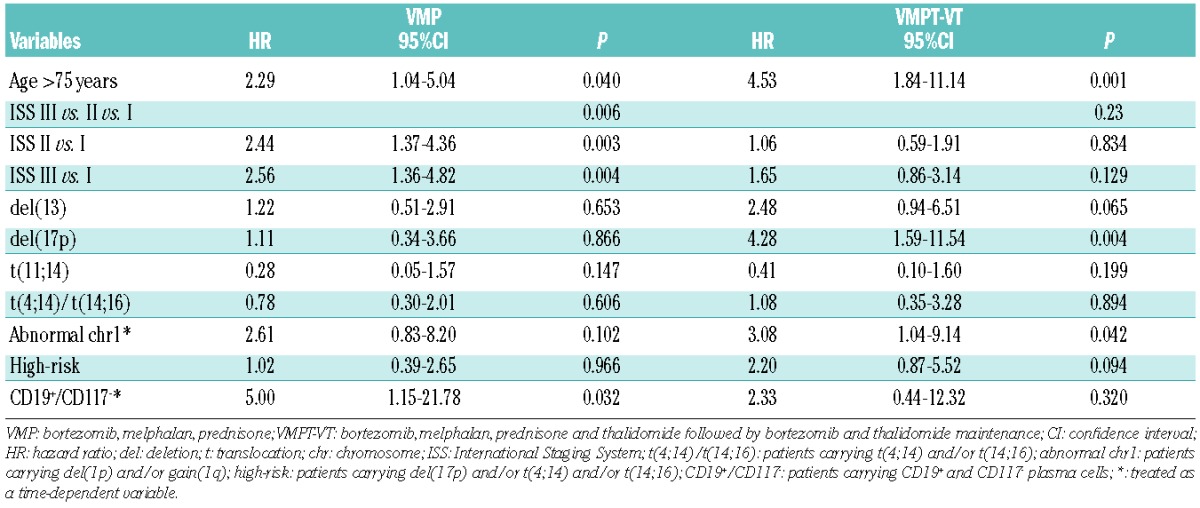
Taking into account that chr1 abnormalities were not equally distributed between the two therapeutic arms, abnormal chr1 and all the other variables were also analyzed separately in order to test for a potential adverse interaction with the thalidomide regimen (Table 3). Abnormal chr1 had a significant adverse impact in the VMPT-VT arm (HR, 3.08; 95% CI, 1.04–9.14; P=0.042), which was not apparent in the VMP arm (HR, 2.61; 95% CI, 0.83–8.20; P=0.102). Moreover, our data suggest that thalidomide impairs survival in patients carrying del(17p) (VMPT-VT arm: HR, 4.28; 95% CI, 1.59–11.54; P=0.004 and VMP arm: HR, 1.11; 95% CI, 0.34–3.66; P=0.866). Conversely, thalidomide had a protective role in patients with CD19+ (VMP arm: HR, 3.89; 95% CI, 1.13–13.38; P=0.031 and VMPT-VT arm: HR, 1.46; 95% CI, 0.29–7.44; P=0.649) or CD19+/CD117− BMPC (VMP arm: HR, 5.00; 95% CI, 1.15–21.78; P=0.032 and VMPT-VT arm: HR, 2.33; 95% CI, 0.44–12.32; P=0.320) and advanced ISS stage (ISS III versus I in the VMP arm: HR, 2.56; 95% CI, 1.36–4.82; P=0.004 and ISS III versus I in the VMPT-VT arm: HR, 1.65; 95% CI, 0.86–3.14; P=0.129). Age significantly affected overall survival in both arms (VMP arm: HR, 2.29; 95% CI, 1.04–5.04; P=0.040 and VMPT-VT arm: HR, 4.53; 95% CI, 1.84–11.14; P=0.001). No significant differences were found between the two arms for the other cytogenetic abnormalities or immunophenotypes.
Table 3.
Univariate Cox analyses for overall survival: impact of baseline clinical and biological characteristics in the VMP or VMPT-VT arm.
Discussion
The introduction of novel agents in the clinical management of MM has led to the need for new risk predictors and although cytogenetic abnormalities represent strong prognostic factors, their real role in risk prediction is still a matter of debate.
Del(13), del(17p), IGH translocations and high-risk chromosomal abnormalities did not show a significant impact on overall survival or progression-free survival of patients enrolled in the VMP versus VMPT-VT trial. This finding confirms and emphasizes the already reported beneficial role of bortezomib, which seems to overcome the negative impact of poor prognostic cytogenetic features.29,31,33 This was demonstrated not just in the study by Palumbo et al.,31 but also in the bortezomib-based trial by Harousseau et al.,34 which showed a similar progression-free survival between cytogenetically defined high-risk and standard-risk patients. Moreover, the Spanish VISTA trial35, comparing MP and VMP, showed that, in the VMP subgroup, there was no statistically significant difference in overall survival between high-risk and standard-risk patients. In line with all these findings, in our series of patients, del(17p), t(4;14) and t(14;16) did not have any impact on clinical outcome, even at the present follow-up. Indeed, a very recent paper from the Mayo Clinic36 set new guidelines for MM treatment defining that: (i) patients with t(4;14) should receive bortezomib as part of induction and maintenance treatment for at least 1 year, in order to overcome the adverse impact of t(4;14) on overall survival; (ii) high-risk patients should receive lenalidomide, bortezomib and dexamethasone; (iii) standard-risk patients can be treated with low-toxicity regimens incorporating lenalidomide and low-dose dexamethasone.
The clinical impact of chr1 abnormalities has so far been evaluated in heterogeneous groups of MM patients, treated with different therapeutic regimens17–19,26–28,37 and gain(1q) and del(1p) were considered so closely related that it is hard to determine their distinct clinical impact.15,38 In this study we referred only to “abnormal chr1”, defined as del(1p) and/or gain (1q), which was present in 50.7% of patients: its poor prognostic impact on overall survival and progression-free survival was more significant than that of del(1p) or gain(1q) considered separately (data not shown).
Logistic regression analysis identified del(13) and t(4;14)/t(14;16) as risk factors of borderline significance for the presence of abnormal chr1, while t(11;14) emerged as a strong protective factor. These data (Table 2, panel A) do not only describe an association, but they highlight a cause-effect relationship between the presence/absence of some chromosomal abnormalities and the onset of an abnormal chr1.
Del(1p) was equally distributed between the two treatment groups, while gain(1q) was more frequent in the VMP treatment group than in the VMPT-VT group. This bias may have occurred since patients were not randomized in the light of cytogenetic characteristics. Chr1 abnormalities were more frequent in the VMP arm and we expected to observe a major negative impact on survival in this group. Interestingly, however, a significant negative impact on overall survival was observed only in the VMPT-VT arm, as shown in Table 3. In other words, although abnormal chr1 was less frequent in the VMPT-VT arm, its negative impact was significant only in this subgroup, probably due to a negative effect of administering thalidomide to these patients.
Smetana et al.29 analyzed several chromosomal abnormalities in 102 patients with relapsed MM treated with bortezomib- or thalidomide-based regimens. They suggested that bortezomib should be preferred to thalidomide in patients with relapsed and/or refractory MM carrying gain(1q), two or more cytogenetic abnormalities and/or del(17p). Our findings show the ability of VMP treatment to overcome the negative prognostic impact of abnormal chr1 in elderly patients with newly diagnosed MM, whereas the addition of thalidomide appears to have a negative effect on overall survival. Recently, the MRC Myeloma IX trial examined the role of thalidomide both as induction and maintenance therapy in patients with del(17p) (n=85). Thalidomide induction was associated with improved response rates, but not with improved overall survival, while, as maintenance therapy, it was associated with impaired survival39. In our cohort of del(17p) patients (n=55), thalidomide impaired overall survival, as shown by the univariate Cox analyses in Table 3. Moreover, Kaplan-Meier analyses also highlighted a negative effect of thalidomide on overall survival in patients with del(17p) (13.5 months in the VMPT group versus 22.5 months in the VMP group, P=0.726), even though this was not statistically significant, probably because of the low frequency of del(17p) (14.6%), whereas thalidomide was observed to have a benefit in patients with a normal(17p) (42.3 months in the VMPT arm versus 31.7 months in the VMP arm, P=0.061). The same trend was also confirmed by Kaplan-Meier analysis for progression-free survival [del(17p) patients: 16.8 months in the VMPT arm versus 19.5 months in the VMP arm, P=0.329; normal(17p) patients: 34.5 months in the VMPT arm versus 23.0 months in the VMP arm, P<0.001). In our study, we could not distinguish between the effects of thalidomide as induction or maintenance therapy, because all the patients in the VMPT arm also received VT maintenance, whereas patients in the VMP arm did not receive thalidomide at all. The detrimental role of thalidomide on overall survival was also evaluated in the whole series of patients in the MRC Myeloma IX trial by Brioli et al.,40 who underlined its negative effect on high-risk patients. Some authors suggest, instead, that thalidomide maintenance is more beneficial in high-risk disease.41 We did not observe any significant difference comparing the high-risk and the standard-risk groups, confirming the benefit of bortezomib administration in high-risk patients independently of thalidomide administration.
The prognostic impact of plasma cell immunophenotype has been broadly investigated by several authors.3,4,7 More recently, CD19 expression on MM plasma cells has been studied and shown to be an adverse prognostic marker,5,7 while CD117 was found to be associated with a favorable outcome.3 In our study, we did not observe any association between clinical outcome and the single expression of CD45, CD20, CD117, or CD56. Mateo et al.3 published the results of an extensive study on 685 newly diagnosed MM patients entered into the GEM 2000 protocol. Their findings indicated that three individual markers, CD19, CD28 and CD117, were prognostically relevant. We observed that CD19+/CD117− patients were characterized by a shorter overall survival, but not progression-free survival. When the analysis was carried out in the two therapeutic arms separately, this combination of antigens only had a negative influence in the VMP arm, suggesting that treatment with thalidomide may overcome its adverse impact.
Recently, it has been argued that the prognostic impact of genetic lesions is modulated over time by changes in the myeloma microenvironment and/or by interactions with new-onset cytogenetic abnormalities.42 For instance, MAF translocations [including t(14;16) and t(14;20)] are associated with a poor prognosis in MM whereas t(14;20) was not linked to disease progression in patients with monoclonal gammopathy of undetermined significance or smoldering MM.43,44 Time dependency of prognostic features was also highlighted by Barlogie et al.45–47 In recent years, the survival of MM patients has been extended from 5 to 10 years or more as a result of autotransplant-supported high-dose melphalan treatment.48,49 This longer follow-up leads to biphasic or triphasic patterns in Kaplan-Meier curves, suggesting that several parameters might govern different time segments of survival outcomes.46 Cytogenetic abnormalities detected by gene expression profiling45, lactate dehydrogenase concentration and calcium levels46, as well as complete response47 have already been described as time-dependent variables. These observations support our findings concerning the time-dependent effects of abnormal chr1, detected by iFISH, and CD19+/CD117− BMPC.
In the multivariate Cox analysis on the whole series of patients, the protective role of thalidomide was not confirmed, while age >75 years, abnormal chr1 and CD19+/CD117− expression were independent predictors for overall survival.
In summary, our findings suggest that abnormal chr1 is an adverse prognostic factor for both overall and progression-free survival in elderly MM patients, as shown in the patients enrolled in the GIMEMA-MM-03-05 trial. CD19+/CD117− BMPC immunophenotype also has an adverse impact on overall survival; however, this antigen combination is rare with respect to abnormal chr1, which affects a large cluster of patients with a major impact on overall survival, as revealed by Cox multivariate analysis. Other genetic abnormalities did not have any impact on overall or progression-free survival, probably due to the administration of bortezomib. However, treatment with thalidomide, even when associated with bortezomib, seems to have a negative effect on patients with abnormal chr1 as well as those with del(17p), whereas it was of benefit in patients with CD19+/CD117− or advanced ISS stage.
Our study is a retrospective and explorative study aimed at better understanding the effect of abnormal chr1 on elderly MM patients treated with novel agents and our results highlight a complex picture of multiple interactions among therapy, risk predictors and time. Although our results need to be confirmed in larger, prospective studies, they may help in the design of future clinical trials.
Acknowledgments
The authors would like to thank the Fondazione Neoplasie Sangue – Onlus (FO.NE.SA.) which supported this research in the form of salaries for the laboratory personnel.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60 [DOI] [PubMed] [Google Scholar]
- 2.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–39 [DOI] [PubMed] [Google Scholar]
- 3.Mateo G, Montalbán MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutiérrez N, et al. PETHEMA Study Group; GEM Study Group. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol. 2008;26(16): 2737–44 [DOI] [PubMed] [Google Scholar]
- 4.Pozdnyakova O, Morgan EA, Li B, Shahsafaei A, Dorfman DM. Patterns of expression of CD56 and CD117 on neoplastic plasma cells and association with genetically distinct subtypes of plasma cell myeloma. Leuk Lymphoma. 2012;53(10):1905–10 [DOI] [PubMed] [Google Scholar]
- 5.Paiva B, Gutiérrez NC, Chen X, Vídriales MB, Montalbán MÁ, Rosiñol L, et al. GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) cooperative. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia. 2012;26(8):1862–9 [DOI] [PubMed] [Google Scholar]
- 6.Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540–7 [DOI] [PubMed] [Google Scholar]
- 7.Bataille R, Jégo G, Robillard N, Barillé-Nion S, Harousseau JL, Moreau P, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica. 2006;91(9):1234–40 [PubMed] [Google Scholar]
- 8.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International Staging System for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20 Erratum in: J Clin Oncol. 2005;23(25):6281 [DOI] [PubMed] [Google Scholar]
- 9.Boyd KD, Ross FM, Chiecchio L, Dagrada GP, Konn ZJ, Tapper WJ, et al. NCRI Haematology Oncology Studies Group. A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia. 2012;26(2):349–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J, et al. Myeloma Working Group. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an International Myeloma Working Group collaborative project. Leukemia. 2013;27(3): 711–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross FM, Ibrahim AH, Vilain-Holmes A, Winfield MO, Chiecchio L, Protheroe RK, et al. UK Myeloma Forum. Age has a profound effect on the incidence and significance of chromosome abnormalities in myeloma. Leukemia. 2005;19(9):1634–42 [DOI] [PubMed] [Google Scholar]
- 12.Avet-Loiseau H, Hulin C, Campion L, Rodon P, Marit G, Attal M, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myélome experience. J Clin Oncol. 2013;31(22):2806–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross FM, Avet-Loiseau H, Ameye G, Gutiérrez NC, Liebisch P, O’Connor S, et al. European Myeloma Network. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97(8):1272–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64(4): 1546–58 [DOI] [PubMed] [Google Scholar]
- 15.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan GJ, Gregory WM, Davies FE, Bell SE, Szubert AJ, Brown JM, et al. National Cancer Research Institute Haematological Oncology Clinical Studies Group. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119(1):7–15 [DOI] [PubMed] [Google Scholar]
- 17.Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109(6):2276–84 [DOI] [PubMed] [Google Scholar]
- 18.Zhan F, Colla S, Wu X, Chen B, Stewart JP, Kuehl WM, et al. CKS1B, overexpressed in aggressive disease, regulates multiple myeloma growth and survival through SKP2- and p27Kip1-dependent and -independent mechanisms. Blood. 2007;109(11): 4995–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leone PE, Walker BA, Jenner MW, Chiecchio L, Dagrada G, Protheroe RK, et al. Deletions of CDKN2C in multiple myeloma: biological and clinical implications. Clin Cancer Res. 2008;14(19):6033–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang H, Jiang A, Qi C, Trieu Y, Chen C, Reece D. Impact of genomic aberrations including chromosome 1 abnormalities on the outcome of patients with relapsed or refractory multiple myeloma treated with lenalidomide and dexamethasone. Leuk Lymphoma. 2010;51(11):2084–91 [DOI] [PubMed] [Google Scholar]
- 21.Boyd KD, Ross FM, Walker BA, Wardell CP, Tapper WJ, Chiecchio L, et al. NCRI Haematology Oncology Studies Group. Mapping of chromosome 1p deletions in myeloma identifies FAM46C at 1p12 and CDKN2C at 1p32.3 as being genes in regions associated with adverse survival. Clin Cancer Res. 2011;17(24):7776–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chng WJ, Gertz MA, Chung TH, Van Wier S, Keats JJ, Baker A, et al. Correlation between array-comparative genomic hybridization-defined genomic gains and losses and survival: identification of 1p31-32 deletion as a prognostic factor in myeloma. Leukemia. 2010;24(4):833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillengass J, Zechmann CM, Nadler A, Hose D, Cremer FW, Jauch A, et al. Gain of 1q21 and distinct adverse cytogenetic abnormalities correlate with increased microcirculation in multiple myeloma. Int J Cancer. 2008;122(12):2871–5 [DOI] [PubMed] [Google Scholar]
- 24.Fonseca R, Van Wier SA, Chng WJ, Ketterling R, Lacy MQ, Dispenzieri A, et al. Prognostic value of chromosome 1q21 gain by fluorescent in situ hybridization and increase CKS1B expression in myeloma. Leukemia. 2006;20(11):2034–40 [DOI] [PubMed] [Google Scholar]
- 25.Chang H, Qi X, Trieu Y, Xu W, Reader JC, Ning Y, Reece D. Multiple myeloma patients with CKS1B gene amplification have a shorter progression-free survival post-autologous stem cell transplantation. Br J Haematol. 2006;135(4):486–91 [DOI] [PubMed] [Google Scholar]
- 26.Nemec P, Zemanova Z, Greslikova H, Michalova K, Filkova H, Tajtlova J, et al. Gain of 1q21 is an unfavorable genetic prognostic factor for multiple myeloma patients treated with high-dose chemotherapy. Biol Blood Marrow Transplant. 2010;16(4):548–54 [DOI] [PubMed] [Google Scholar]
- 27.Klein U, Jauch A, Hielscher T, Hillengass J, Raab MS, Seckinger A, et al. Chromosomal aberrations +1q21 and del(17p13) predict survival in patients with recurrent multiple myeloma treated with lenalidomide and dexamethasone. Cancer. 2011;117(10): 2136–44 [DOI] [PubMed] [Google Scholar]
- 28.Chang H, Trieu Y, Qi X, Jiang NN, Xu W, Reece D. Impact of cytogenetics in patients with relapsed or refractory multiple myeloma treated with bortezomib: adverse effect of 1q21 gains. Leuk Res. 2011;35(1):95–8 [DOI] [PubMed] [Google Scholar]
- 29.Smetana J, Berankova K, Zaoralova R, Nemec P, Greslikova H, Kupska R, et al. Gain(1)(q21) is an unfavorable genetic prognostic factor for patients with relapsed multiple myeloma treated with thalidomide but not for those treated with bortezomib. Clin Lymphoma Myeloma Leuk. 2013;13(2):123–30 [DOI] [PubMed] [Google Scholar]
- 30.Grzasko N, Hus M, Pluta A, Jurczyszyn A, Walter-Croneck A, Morawska M, et al. Additional genetic abnormalities significantly worsen poor prognosis associated with 1q21 amplification in multiple myeloma patients. Hematol Oncol. 2013;31(1):41–8 [DOI] [PubMed] [Google Scholar]
- 31.Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101–9 [DOI] [PubMed] [Google Scholar]
- 32.Palumbo A, Bringhen S, Larocca A, Rossi D, Di Raimondo F, Magarotto V, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32(7):634–40 [DOI] [PubMed] [Google Scholar]
- 33.Tuchman SA, Lonial S. High-risk multiple myeloma: does it still exist¿ Clin Lymphoma Myeloma Leuk. 2011;11(Suppl 1):S70–6 [DOI] [PubMed] [Google Scholar]
- 34.Harousseau JL, Avet-Loiseau H, Attal M, Marit G, Caillot D, Hulin C, et al. High complete and very good partial response rates with bortezomib-dexamethasone as induction prior to ASCT in newly diagnosed patients with high-risk myeloma: results of the IFM2005-01 phase 3 trial. Blood (ASH Annual Meeting Abstracts). 2009;114: 353 [Google Scholar]
- 35.Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–66 [DOI] [PubMed] [Google Scholar]
- 36.Bergsagel PL, Chesi M. V. Molecular classification and risk stratification of myeloma. Hematol Oncol. 2013;31(Suppl 1):38–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Min HJ, Park HK, Oh B, Kim TY, She CJ, et al. Korea Multiple Myeloma Working Party. Increased copy number of the interleukin-6 receptor gene is associated with adverse survival in multiple myeloma patients treated with autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):810–20 [DOI] [PubMed] [Google Scholar]
- 38.Chang H, Qi X, Jiang A, Xu W, Young T, Reece D. 1p21 deletions are strongly associated with 1q21 gains and are an independent adverse prognostic factor for the outcome of high-dose chemotherapy in patients with multiple myeloma. Bone Marrow Transplant. 2010;45(1):117–21 [DOI] [PubMed] [Google Scholar]
- 39.Boyd KD, Ross FM, Tapper WJ, Chiecchio L, Dagrada G, Konn ZJ, et al. NCRI Haematology Oncology Studies Group. The clinical impact and molecular biology of del(17p) in multiple myeloma treated with conventional or thalidomide-based therapy. Genes Chromosomes Cancer. 2011;50(10): 765–74 [DOI] [PubMed] [Google Scholar]
- 40.Brioli A, Kaiser MF, Pawlyn C, Wu P, Gregory WM, Owen R, et al. Biologically defined risk groups can be used to define the impact of thalidomide maintenance therapy in newly diagnosed multiple myeloma. Leuk Lymphoma. 2013;54(9):1975–81 [DOI] [PubMed] [Google Scholar]
- 41.Barlogie B, Pineda-Roman M, van Rhee F, Haessler J, Anaissie E, Hollmig K, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112(8):3115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–48 [DOI] [PubMed] [Google Scholar]
- 43.Ross FM, Chiecchio L, Dagrada G, Protheroe RK, Stockley DM, Harrison CJ, et al. The t(14;20) is a poor prognostic factor in myeloma but is associated with long-term stable disease in monoclonal gammopathies of undetermined significance. Haematologica. 2010;95(7):1221–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barlogie B, Anaissie E, van Rhee F, Shaughnessy JD, Jr, Szymonifka J, Hoering A, et al. Reiterative survival analyses of total therapy 2 for multiple myeloma elucidate follow-up time dependency of prognostic variables and treatment arms. J Clin Oncol. 2010;28(18):3023–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barlogie B, Bolejack V, Schell M, Crowley J. Prognostic factor analyses of myeloma survival with intergroup trial S9321 (INT 0141): examining whether different variables govern different time segments of survival. Ann Hematol. 2011;90(4):423–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoering A, Crowley J, Shaughnessy JD, Jr, Hollmig K, Alsayed Y, Szymonifka J, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114(7): 1299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. InterGroupe Francophone du Myélome. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349(26):2495–502 Erratum in: N Engl J Med. 2004;350(25):2628 [DOI] [PubMed] [Google Scholar]
- 48.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356(11):1110–20 [DOI] [PubMed] [Google Scholar]
- 49.San Miguel JF, Gutiérrez NC, Mateo G, Orfao A. Conventional diagnostics in multiple myeloma. Eur J Cancer. 2006;42(11): 1510–9 [DOI] [PubMed] [Google Scholar]


