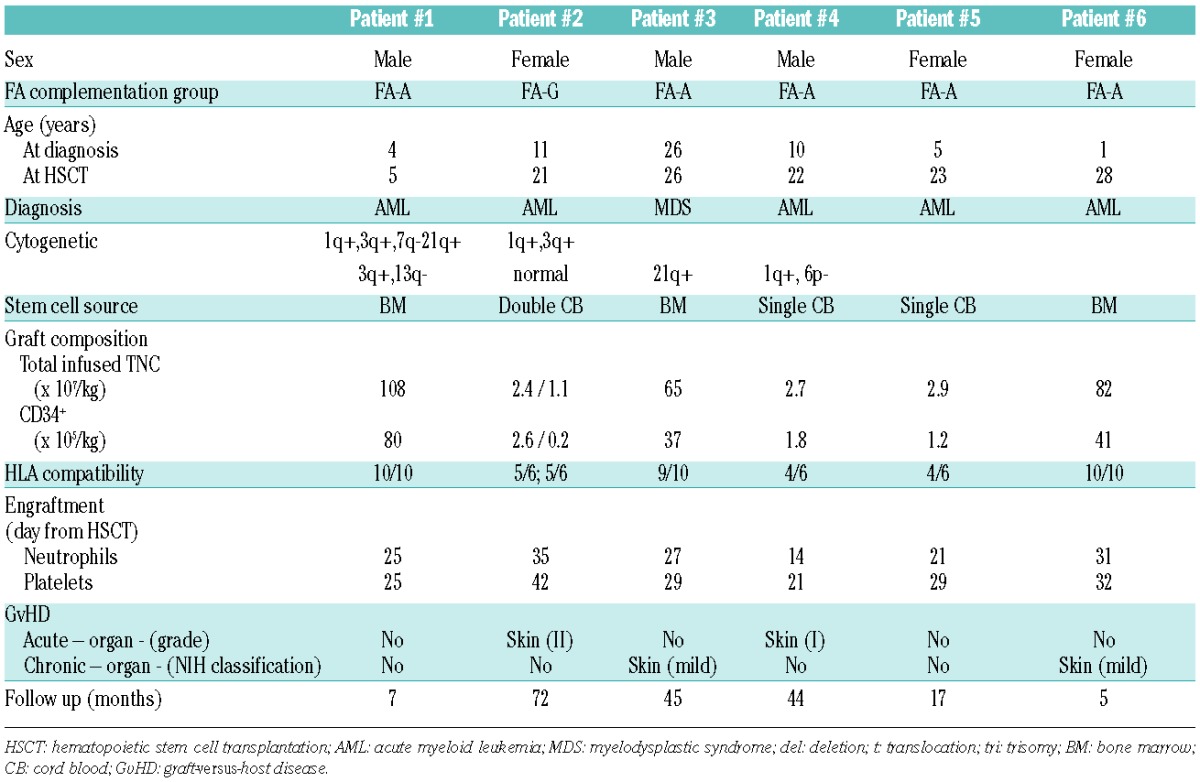
The natural history of Fanconi anemia is characterized by progressive marrow failure and evolution to acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), and by an increased rate of solid tumors.1–4 AML and MDS in this setting are associated with a dismal prognosis. Although allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative approach for AML/MDS in the FA patient, it has been associated with significant treatment-related mortality.5 Furthermore, induction chemotherapy for AML in FA patients is also associated with significant toxicity and a prolonged period of aplasia. The use of sequential chemotherapy and HSCT has been described in non-FA patients not in remission.6 We report the outcome of 6 FA patients who received a sequential regimen strategy because of clonal evolution. From August 2006 to December 2011, 6 consecutive patients with FA (Table 1) who received sequential chemotherapy and reduced intensity conditioning (RIC) before HSCT for clonal evolution in 4 French institutions were reviewed. Five patients had AML (3 high-risk and 2 standard-risk according to Medical Research Council criteria) and one had a high-risk MDS according to the International Prognostic Scoring System. The sequential strategy consisted of pre-transplant chemotherapy with fludarabine 30 mg/m2/d for five days and cytarabine 1 gr/m2×2/d for five days with granulocyte-colony stimulating factor injections (FLAG). This was followed three weeks later by an RIC: 4 days cyclophosphamide 10 mg/kg (low-dose cyclophosphamide because of FA), 4 days fludarabine 30 mg/m2, 2 days anti-thymocyte globulin (3.75 mg/kg) and TBI (2 Gy) delivered during chemotherapy-induced aplasia. All patients were still in aplasia when they were transplanted. The source of stem cells was cord blood in 3 patients (2 cord blood units) and bone marrow for the other 3. Graft-versus-host disease (GvHD) prophylaxis consisted of cyclosporine (CSA) plus MMF (except 1 patient who received CSA plus methotrexate). Median age of the patients at HSCT was 20.5 years (range 5–28 years). All patients engrafted. Median time to engraftment was 21 days (range 14–35 days) for neutrophils and 29 days for platelets (range 21–42 days). Donor chimerism was complete at Day 100 for 5 patients. The sequential regimen was well tolerated. Three patients developed bacteremia (Staphylococcus, Enterobacter, and Candida) at one, three and one months after HSCT, respectively. One patient developed a diarrhea to Campylobacter jejuni one month after HSCT and another patient underwent surgery for chronic maxillary sinusitis six months after HSCT. Acute GvHD (one grade 1 and one grade 2) occurred in 2 patients; both responded to steroid therapy. Chronic GvHD occurred in 2 patients (only skin was involved, corresponding to mild cGvHD according to the NIH working group definition7). After a median follow up of 28 months (range 5–72 months), all patients are alive in complete remission from clonal evolution. Because of the usual very poor outcome of such patients, we believe this study supports the use of such a sequential strategy (FLAG and RIC HSCT) in FA patients with clonal evolution.
Table 1.
Patients’ and transplant characteristics.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.MacMillan ML, Wagner JE. Haematopoeitic cell transplantation for Fanconi anaemia - when and how¿ Br J Haematol. 2011;149(1):14–21 [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101(3):822–6 [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105(1):67–73 [DOI] [PubMed] [Google Scholar]
- 4.Alter BP, Greene MH, Velazquez I, Rosenberg PS. Cancer in Fanconi anemia. Blood. 2003;101(5):2072. [DOI] [PubMed] [Google Scholar]
- 5.Guardiola P, Kurre P, Vlad A, Cayuela JM, Esperou H, Devergie A, et al. Effective graft-versus-leukaemia effect after allogeneic stem cell transplantation using reduced-intensity preparative regimens in Fanconi anaemia patients with myelodysplastic syndrome or acute myeloid leukaemia. Br J Haematol. 2003;122(5):806–9 [DOI] [PubMed] [Google Scholar]
- 6.Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675–87 [DOI] [PubMed] [Google Scholar]
- 7.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56 [DOI] [PubMed] [Google Scholar]


