Abstract
Purpose
To examine the associations of neighborhood socioeconomic deprivation and triple-negative breast cancer (TNBC) subtype with causes of death (breast cancer [BC]-specific and non-BC-specific) among non-metastatic invasive BC patients.
Methods
We identified 3,312 patients younger than 75 years (mean age 53.5 years; 621 [18.8%] TNBC) with first primary BC treated at an academic medical center from 1999–2010. We constructed a census-tract-level socioeconomic deprivation index using the 2000 U.S. Census data and performed a multilevel competing-risk analysis to estimate the hazard ratios (HR) and 95% confidence intervals (CI) of BC-specific and non-BC-specific mortality associated with neighborhood socioeconomic deprivation and TNBC subtype. The adjusted models controlled for patient sociodemographics, health behaviors, tumor characteristics, comorbidity, and cancer treatment.
Results
With a median 62-month follow-up, 349 (10.5%) patients died; 233 died from BC. In the multivariate models, neighborhood socioeconomic deprivation was independently associated with non-BC-specific mortality (the most- vs. the least-deprived quartile: HR=2.98, 95% CI=1.33–6.66); in contrast, its association with BC-specific mortality was explained by the aforementioned patient-level covariates, particularly sociodemographic factors (HR=1.15, 95% CI=0.71–1.87). TNBC subtype was independently associated with non-BC-specific mortality (HR=2.15; 95% CI=1.20–3.84), while the association between TNBC and BC-specific mortality approached significance (HR=1.42; 95% CI=0.99–2.03, P=0.057).
Conclusions
Non-metastatic invasive BC patients who lived in more socioeconomically deprived neighborhoods were more likely to die as a result of causes other than breast cancer compared with those living in the least socioeconomically deprived neighborhoods. TNBC was associated with non-BC-specific mortality but not BC-specific mortality.
Keywords: breast cancer, subtype, mortality, neighborhood socioeconomic deprivation, multilevel analysis, competing risk
Introduction
Breast cancer (BC) prognosis has markedly improved over the past three decades, mainly due to the widespread use of screening mammography and improvements in treatment [1]. Nevertheless, socioeconomic disparities in BC prognosis persist. BC prognosis is worse among women who live in communities with greater socioeconomic deprivation [2–10]. This prognostic disparity has been attributed to patient and clinic factors, including differences in the incidence of tumors characterized by pathologically and biologically aggressive phenotypes, the prevalence of obesity and other comorbid conditions, health-risk behaviors, access to treatment, and quality of care received [4, 11, 12].
Several studies have reported that women living in neighborhoods with greater socioeconomic deprivation were more likely than those living in more affluent areas to have been diagnosed with estrogen-receptor (ER)-negative BC [8, 13], and particularly with triple-negative BC (TNBC) [14], which is characterized by ER-negative, progesterone-receptor (PR)-negative, and human epidermal growth factor receptor-2 (HER2)-negative tumors. TNBC is associated with early recurrence and poor survival due to lack of specific targets for commonly used adjuvant therapies [15]. It remains unclear whether TNBC explains the observed worse prognosis of BC patients living in neighborhoods with greater socioeconomic deprivation.
In addition, women with non-metastatic invasive BC, especially older survivors are more likely to die from causes other than BC, including cardiovascular disease and other comorbid conditions [16, 17]. Although neighborhood socioeconomic factors and TNBC subtype have been associated with overall and BC-specific mortality among women with BC [2–10], it remains unknown if neighborhood socioeconomic deprivation and TNBC subtype also contribute to death from diseases other than breast cancer, especially in women with non-metastatic invasive BC.
Disentangling the impact of neighborhood socioeconomic deprivation and TNBC on causes of death can help us develop effective interventions at multiple levels to reduce racial and geographic disparities and improve BC prognosis. Thus, using a multilevel competing-risk approach, we evaluated and compared the associations of neighborhood socioeconomic deprivation and TNBC subtype with BC-specific and non-BC-specific mortality among non-metastatic invasive BC survivors.
Methods
Study Sample
From a prospective tumor registry of patients diagnosed and treated at the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, we identified women with first-primary BC diagnosed between March 1999 and March 2010, who had no prior cancer history and were followed through March 31, 2011 (n=5,339). For each patient, we obtained sociodemographic, health behavior, and clinical information from the medical record. We excluded patients aged 75 or older (n=522) and patients whose residential addresses could not be geocoded (n=338). Given the aim of our study, we also excluded patients with in-situ carcinoma (n=982) due to their extremely low BC-specific mortality with a 10-year estimate <2.5% [18] and patients with metastatic BC (n=257) due to their extremely high BC-specific mortality with a 5-year estimate >73% [19]. Overall, 1894 patients meeting one or more of these criteria were excluded from the analysis. This study was approved by the Institutional Review Board at Washington University School of Medicine.
ER and PR status was determined using immunohistochemistry and considered positive if an Allred score was above 2 or more than 1% of tumor cells showed nuclear staining [20]. Tumors were designated as being HER2+ by FISH 2+/3+ or immunohistochemistry staining in more than 10% of tumor cells [21]. We further excluded 133 of 3,445 otherwise eligible patients with insufficient receptor-status information. Thus, 3,312 BC patients were included in this study, and their tumors were classified as TNBC or non-TNBC.
Neighborhood Socioeconomic Deprivation Index
Using Geographic Information Systems (ArcGIS 9.3, ESRI, Redland, CA), patients’ residential addresses at diagnosis were geocoded to obtain their census tracts. We identified 867 residential census tracts that contained at least one patient (average 4 patients (range 1–21) per tract).
We constructed a composite census-tract-level socioeconomic deprivation index using a principal components common factor analysis with varimax rotation of 21 variables from 2000 U.S. Census. Based on the literature [22, 23], these Census variables were selected from six domains, including education, occupation, housing conditions, income and poverty, racial composition, and residential stability. The first common factor included seven variables with significant factor loadings and explained 43.7% of the total variance of the 21 census variables. These seven variables, including % civilian labor force unemployed, % households with >=1 person/room, % households female-headed with dependent children, % households on public assistance, % households without vehicle, % population below federal poverty line, and % African Americans, had a high internal consistency (Cronbach alpha=0.95). The variables were standardized and weighted by factor loading coefficients, to compute a neighborhood socioeconomic deprivation index, as described elsewhere [22, 23]. The socioeconomic deprivation index was categorized into quartiles according to its distribution in our sample.
Causes of Death
Causes of death were ascertained through the linkage to the National Death Index. Four underlying causes of death were examined, including BC-specific death (ICD10, C50), circulatory system diseases (ICD10, I00-I99), cancers other than BC (ICD10, C00-D48 excluding C50), and other causes (all other ICD10 codes). To ensure having enough statistical power, we pooled together all causes of deaths other than BC for the analysis. In addition to all-cause mortality as an endpoint, we assessed BC-specific and non-BC-specific mortality as separate endpoints. Person-years were calculated from the date of BC diagnosis until date of death or end of follow-up (March 31, 2011), whichever occurred first.
Statistical Analysis
Chi-square tests were used to compare patient characteristics stratified by quartiles of neighborhood socioeconomic deprivation. We used a cumulative incidence function to describe cause-specific survival by the quartiles of neighborhood socioeconomic deprivation index and by tumor subtypes and the Gray’s test to test for cause-specific survival differences [24, 25]. Multilevel Cox proportional hazards models with gamma frailty [26] were applied to estimate the fixed effects of neighborhood-level socioeconomic deprivation and tumor subtypes on all-cause mortality. Models were controlled for demographics, including age at diagnosis (<45, 45–54, 55–64, or 65–74 years), race (white, African American, or others), marital status (married/partnered or unmarried/unpartnered), type of insurance (private, public, or uninsured); tumor characteristics, including cancer stage (I, II, III), tumor grade (well, moderately, or poorly differentiated), tumor subtypes, comorbidity (coded for analysis by level of decompensation as none/mild, moderate, or severe) using the ACE-27 [27], which includes numerous medical/psychiatric conditions and behaviors with prognostic significance (such as obesity and substance abuse); treatments, including hormone therapy (yes/no), trastuzumab therapy (yes/no), chemotherapy (yes/no), radiotherapy (yes/no), surgical treatment (mastectomy, lumpectomy, or no surgical treatment); health behaviors, including alcohol use (none or former drinker, current drinker), and smoking status (never or former smoker, current smoker). To identify the factors that account for the prognostic difference across neighborhood socioeconomic deprivation and tumor subtypes, the analysis was controlled for four blocks of the aforementioned covariates, individually and then in combination. Tests for trend were performed by using the median value for each quartile of the neighborhood socioeconomic deprivation index as a continuous variable in the multivariable model. To determine whether neighborhood socioeconomic deprivation and tumor subtypes were differentially associated with BC-specific mortality and non-BC mortality, causes of death were treated as competing risks in the multilevel Cox proportional hazards model. Specifically, we estimated hazard ratios (HRs) and their 95% confidence intervals (CI) of death due to BC or to other causes using the approach described by Lunn and McNeil [28], and used likelihood-ratio tests for heterogeneity [29]. Since the competing risk approach applies an internal stratification, it does not decrease the study sample size and statistical power [28].
Data management and the analysis of cumulative survival probabilities with competing risk were conducted using SAS System (Ver.9.2, SAS Institute Inc., Cary, NC). Multilevel Cox proportional hazards regression analyses were performed in R statistical package (Ver.2.10.1, The R Foundation for Statistical Computing). Two-sided P<0.05 was considered statistically significant.
Results
Among 3,312 women included in analysis, 621 (18.8%) had TNBC, 2,420 (73.1%) were white, 812 (24.5%) were African American, 1,929 (58.2%) were married or partnered, 1,221 (36.9%) were current alcohol drinkers, and 1,224 (37.0%) were current smokers. The mean age was 53.5 years (range 17–74). Only 209 (6.3%) patients were uninsured. Among 3,103 women with health insurance, 37.8% were insured by public health plans. Of all patients, 42.3% had poorly-differentiated tumors, and 58.3% were diagnosed with stages II-III tumors. Severe comorbidity was observed in 4.8% of patients. Overall, 1.9% of patients did not receive definitive surgical treatment for BC, 44.1% were treated with mastectomy, and 53.6% received breast-conserving surgery. Regarding adjuvant therapy, 62.3% underwent anti-estrogen therapy, 5.6% received trastuzumab therapy, 62.5% received chemotherapy, and 66.3% received radiation therapy.
Table 1 shows patient characteristics by neighborhood socioeconomic deprivation. Compared with women living in neighborhoods with less socioeconomic deprivation, women living in neighborhoods with greater socioeconomic deprivation were more likely to have TNBC, be African American, be unmarried/unpartnered, have public health insurance coverage, have more severe comorbidity, have received chemotherapy, and have tumors characterized by more advanced stage and poor differentiation (each P<0.05). However, women living in neighborhoods with greater socioeconomic deprivation were less likely to have received surgical treatment and hormone therapy (each P<0.05).
Table 1.
Individual characteristics of women with non-metastatic invasive breast cancer (N=3,312) stratified by neighborhood socioeconomic deprivation.
| Variable | Neighborhood Socioeconomic Deprivation a
|
P b | |||
|---|---|---|---|---|---|
| Q1 (n=827) | Q2 (n=828) | Q3 (n=826) | Q4 (n=831) | ||
| Triple-Negative Subtype | <0.001 | ||||
| Yes | 126 (15.2) | 132 (15.9) | 144 (17.4) | 219 (26.4) | |
| No | 701 (84.8) | 696 (84.1) | 682 (82.6) | 612 (73.7) | |
| Age (Years) | 0.108 | ||||
| <45 | 195 (23.6) | 178 (21.5) | 146 (17.7) | 172 (20.7) | |
| 45–54 | 269 (32.5) | 253 (30.6) | 268 (32.5) | 286 (34.4) | |
| 55–64 | 226 (27.3) | 256 (30.9) | 251 (30.4) | 238 (28.6) | |
| 65–74 | 137 (16.6) | 141 (17.0) | 161 (19.5) | 135 (16.3) | |
| Race | <0.001 | ||||
| White | 776 (93.8) | 763 (92.2) | 677 (82.0) | 204 (24.6) | |
| AA | 21 (2.5) | 45 (5.4) | 131 (15.9) | 615 (74.0) | |
| Others | 30 (3.6) | 20 (2.4) | 18 (2.2) | 12 (1.4) | |
| Marital | <0.001 | ||||
| Married/Partnered | 623 (75.3) | 566 (68.4) | 463 (56.1) | 277 (33.3) | |
| Unmarried/Unpartnered | 204 (24.7) | 262 (31.6) | 363 (44.0) | 554 (66.7) | |
| Insurance | <0.001 | ||||
| Private/managed care | 572 (69.2) | 534 (64.5) | 464 (56.2) | 360 (43.3) | |
| Medicaid/Medicare/public | 203 (24.6) | 242 (29.3) | 310 (37.5) | 418 (50.3) | |
| Uninsured | 52 (6.3) | 52 (6.3) | 52 (6.3) | 53 (6.4) | |
| Stage | <0.001 | ||||
| I | 381 (46.1) | 377 (45.5) | 336 (40.7) | 286 (34.4) | |
| II | 334 (40.4) | 343 (41.4) | 361 (43.7) | 403 (48.5) | |
| III | 112 (13.5) | 108 (13.0) | 129 (15.6) | 142 (17.1) | |
| Grade | <0.001 | ||||
| Well differentiated | 198 (23.9) | 189 (22.8) | 204 (24.7) | 146 (17.6) | |
| Moderately | 324 (39.2) | 310 (37.4) | 303 (36.7) | 237 (28.5) | |
| Poorly | 305 (36.9) | 329 (39.7) | 319 (38.6) | 448 (53.9) | |
| Comorbidity | <0.001 | ||||
| Mild/no | 661 (89.3) | 632 (86.5) | 622 (85.6) | 605 (79.1) | |
| Moderately | 49 (6.6) | 59 (8.1) | 66 (9.1) | 110 (14.4) | |
| Severe | 30 (4.1) | 40 (5.5) | 39 (5.4) | 50 (6.5) | |
| Missing | 87 | 97 | 99 | 66 | |
| Hormone therapy | <0.001 | ||||
| Yes | 562 (68.8) | 526 (64.4) | 535 (66.0) | 439 (53.3) | |
| No | 255 (31.2) | 291 (35.6) | 276 (34.0) | 384 (46.7) | |
| Missing | 10 | 11 | 15 | 8 | |
| Trastuzumab therapy | 0.475 | ||||
| Yes | 55 (6.7) | 41 (5.0) | 44 (5.3) | 46 (5.5) | |
| No | 772 (93.4) | 787 (95.1) | 782 (94.7) | 785 (94.5) | |
| Chemotherapy | 0.017 | ||||
| Yes | 512 (62.1) | 502 (60.9) | 501 (61.2) | 555 (67.5) | |
| No | 312 (37.9) | 322 (39.1) | 318 (38.8) | 267 (32.5) | |
| Missing | 3 | 4 | 7 | 9 | |
| Radiotherapy | 0.401 | ||||
| Yes | 549 (66.9) | 565 (68.8) | 548 (67.0) | 533 (64.8) | |
| No | 272 (33.1) | 256 (31.2) | 270 (33.0) | 289 (35.2) | |
| Missing | 6 | 7 | 8 | 9 | |
| Surgery | 0.001 | ||||
| Mastectomy | 382 (46.5) | 375 (45.5) | 363 (44.15) | 342 (41.3) | |
| Lumpectomy | 432 (52.6) | 440 (53.3) | 446 (54.1) | 457 (55.1) | |
| None | 8 (1.0) | 10 (1.2) | 15 (1.8) | 30 (3.6) | |
| Missing | 5 | 3 | 2 | 2 | |
| Alcohol use | <0.001 | ||||
| No or former use | 472 (57.7) | 501 (61.3) | 526 (64.2) | 552 (67. 6) | |
| Current use | 346 (42.3) | 316 (38.7) | 294 (35.9) | 265 (32.4) | |
| Missing | 9 | 11 | 6 | 14 | |
| Smoking status | <0.001 | ||||
| Non-smoker/former | 482 (66.2) | 444 (60.0) | 432 (57.2) | 406 (53.1) | |
| Current smoker | 246 (33.8) | 296 (40.0) | 323 (42.8) | 359 (46.9) | |
| Missing | 99 | 88 | 71 | 66 | |
Q1 denotes the least socioeconomically deprived quartile and Q4 denotes the most socioeconomically deprived quartile; there were 242 census tracts in Q1, 230 in Q2, 236 in Q3, and 159 in Q4.
Chi-square tests.
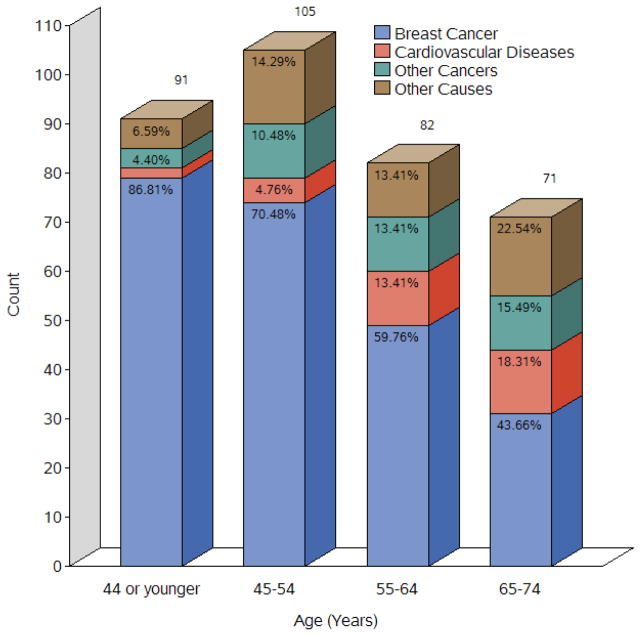
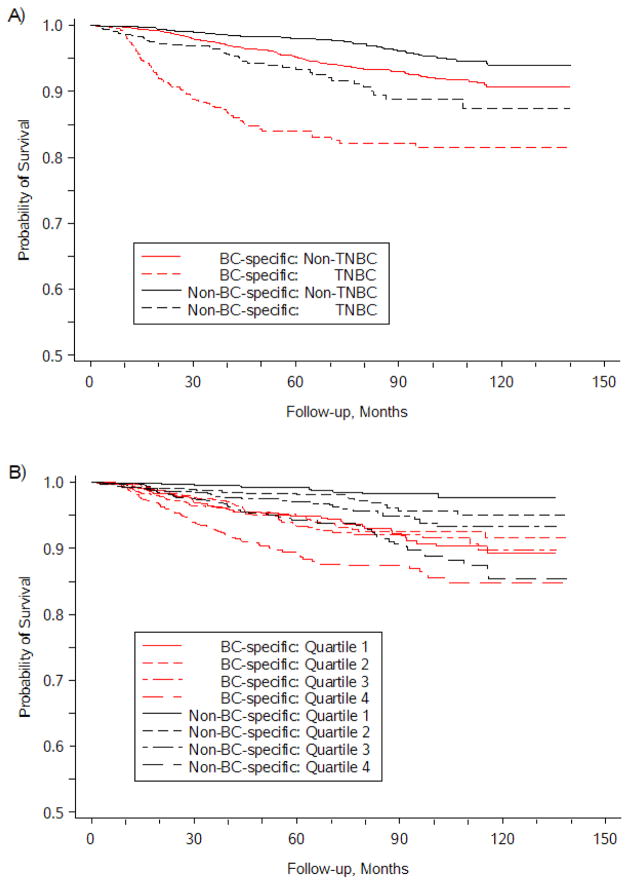
Overall 349 (10.5%) died during a median follow-up of 62 months (range 2–140). Of them, 66.8% were due to BC, 8.9% to circulatory system diseases, 10.6% to other cancers, and 13.8% to other causes. Diseases other than BC are the major cause of death in older patients (65–74 years), while greater than half of younger patients died from BC (Figure 1). The 5-year BC-specific survival rate for the entire cohort was 93.2%, with 84.0% in TNBC patients and 95.2% in non-TNBC patients. The 5-year non-BC-specific survival rate was 93.3% in TNBC patients and 98.0% in non-TNBC patients. The differences in BC-specific and non-BC survival probabilities were statistically significant between TNBC and non-TNBC (log-rank test, P<0.001, Figure 2A). The 5-year BC-specific survival rate was 89.5% in women living in neighborhoods with the greatest socioeconomic deprivation and 94.8% in women living in neighborhoods with the least socioeconomic deprivation. Meanwhile, the 5-year non-BC-specific survival rate was 97.2%, with an estimate of 94.3% in women living in neighborhoods with the greatest socioeconomic deprivation and 99.2% in women living in neighborhoods with the least socioeconomic deprivation. The differences in BC-specific and non-BC survival probabilities were statistically significant across the quartiles of neighborhood socioeconomic deprivation (log-rank test, P<0.001 for each, Figure 2B).
Figure 1.
Causes of death of women with non-metastatic invasive breast cancer, by age groups (N=3,312).
Figure 2.
Competing-risk Kaplan-Meier survival curves for breast cancer patients by: A) TNBC status, and B) socioeconomic deprivation quartiles. Log-rank tests indicated statistically significant differences by TNBC status and by level of socioeconomic deprivation (both P<0.001).
Table 2 shows HRs of all-cause mortality and competing risks of causes of death associated with neighborhood socioeconomic deprivation and tumor subtypes. In the bivariate analysis, neighborhood socioeconomic deprivation was significantly associated with all-cause mortality (the most vs. the least deprived: HR=2.19, 95% CI=1.62–2.97, Ptrend<0.001), BC-specific mortality (HR=1.58, 95% CI=1.11–2.24, Ptrend<0.001) and non-BC-specific mortality (HR=5.34, 95% CI=2.71–10.50, Ptrend<0.001). The multivariate analysis adjusted for all four blocks of covariates showed that neighborhood socioeconomic deprivation was still significantly associated with non-BC-specific mortality (HR=2.98, 95% CI=1.33–6.66, Ptrend=0.023), and the association with all-cause mortality approached significance (HR=1.41, 95% CI=0.94–2.13, Ptrend=0.055). Accounting for four individual blocks of covariates, we found that the association between neighborhood socioeconomic deprivation and BC-specific mortality was attenuated by individual-level sociodemographic factors to a nonsignificant level (Supplementary Table 1). However, adjustments for other individual-level factors shown in Supplementary Table 1 – for tumor characteristics and comorbidity (Model II), treatment (Model III), and selected health behaviors (Model IV) – did not significantly reduce the association between neighborhood socioeconomic deprivation and non-BC-specific mortality. The difference in the effects of neighborhood socioeconomic deprivation on BC-specific and non-BC-specific mortality was statistically significant (Pheterogeneity=0.007) in the multivariate competing risk analysis (Table 2). These findings indicate that neighborhood socioeconomic deprivation was associated with both BC-specific and non-BC-specific mortality in the bivariate models, independent of tumor subtypes (Table 2). As shown in Supplemental Table 1, the association between neighborhood socioeconomic deprivation and BC-specific mortality was explained by individual-level sociodemographic factors, while the association with non-BC-mortality was not explained by individual-level sociodemographic, clinical, or behavioral factors examined.
Table 2.
All-cause mortality and competing risks of breast cancer (BC)-specific mortality and non-BC-specific mortality associated with neighborhood socioeconomic deprivation and tumor subtypes (TNBC vs. non-TNBC).
| Person-Years | All-cause Mortality
|
Competing Risk Model
|
|||||
|---|---|---|---|---|---|---|---|
| BC-specific mortality
|
Non-BC-specific mortality
|
||||||
| Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | ||
| Bivariate models | |||||||
| Neighborhood Socioeconomic Deprivation Quartile | |||||||
| 1st (lowest) | 4681 | 61 | 1.00 | 51 | 1.00 | 10 | 1.00 |
| 2nd | 4570 | 65 | 1.08 (0.76–1.53) | 44 | 0.87 (0.58–1.30) | 21 | 2.14 (1.01–4.56) |
| 3rd | 4476 | 82 | 1.37 (0.99–1.92) | 52 | 1.04 (0.70–1.53) | 30 | 3.11 (1.52–6.37) |
| 4th (highest) | 4329 | 141 | 2.19 (1.62–2.97) | 86 | 1.58 (1.11–2.24) | 55 | 5.34 (2.71–10.5) |
| Ptrenda | <0.001 | <0.001 | <0.001 | ||||
| Pheterogeneityb | - | 0.010 | |||||
| Tumor subtype | |||||||
| Non-TNBC | 15137 | 218 | 1.00 | 143 | 1.00 | 75 | 1.00 |
| TNBC | 2919 | 131 | 2.86 (2.30–3.56) | 90 | 3.04 (2.33–3.97) | 41 | 2.52 (1.71–3.70) |
| Pheterogeneity | - | 0.655 | |||||
| Multivariate models | |||||||
| Neighborhood Socioeconomic Deprivation Quartile | |||||||
| 1st (lowest) | 4681 | 61 | 1.00 | 51 | 1.00 | 10 | 1.00 |
| 2nd | 4570 | 65 | 0.97 (0.67–1.41) | 44 | 0.84 (0.55–1.28) | 21 | 1.70 (0.79–3.68) |
| 3rd | 4476 | 82 | 1.12 (0.78–1.60) | 52 | 0.88 (0.58–1.34) | 30 | 2.48 (1.18–5.18) |
| 4th (highest) | 4329 | 141 | 1.41 (0.94–2.13) | 86 | 1.15 (0.71–1.87) | 55 | 2.98 (1.33–6.66) |
| Ptrend | 0.055 | 0.300 | 0.023 | ||||
| Pheterogeneity | - | 0.007 | |||||
| Tumor subtype | |||||||
| Non-TNBC | 15137 | 218 | 1.00 | 143 | 1.00 | 75 | 1.00 |
| TNBC | 2919 | 131 | 1.56 (1.15–2.12) | 90 | 1.42 (0.99–2.03) | 41 | 2.15 (1.20–3.84) |
| Pheterogeneity | - | 0.655 | |||||
Multivariate models were controlled for age, race, marital status, health insurance coverage, tumor stage, tumor grade, comorbidity, hormone therapy, trastuzumab therapy, chemotherapy, radiotherapy, type of surgery, alcohol use, and smoking status;
P value of trend test indicates if there is a significant trend in the effect of neighborhood socioeconomic deprivation;
P value of heterogeneity test indicates if there is a difference in the effects of neighborhood socioeconomic deprivation and TNBC subtype on BC-specific vs. non-BC-specific mortality in the competing-risk models.
Meanwhile, TNBC patients had significantly higher risk of all-cause mortality in the multivariable analysis (HR=1.56, 95% CI=1.15–2.12) compared with non-TNBC patients. TNBC subtype was significantly associated with both non-BC mortality (HR=2.52; 95% CI=1.71–3.70) and BC-specific mortality (HR=3.04; 95% CI=2.33–3.97). Adjusted for all covariates, TNBC subtype was still significantly associated with non-BC mortality (HR=2.15; 95% CI=1.20–3.84), and the association between TNBC and BC-specific mortality approached significance (HR=1.42; 95% CI=0.99–2.03, P=0.057). The association between tumor subtype and mortality did not vary significantly across causes of death (Pheterogeneity=0.655).
Additionally, we found substantial racial disparity in BC-specific and non-BC-specific mortality. Compared to white women, African American women with non-metastatic invasive BC had higher risk in both BC-mortality (HR=1.87, 95% CI=1.43–2.45) and non-BC mortality (HR=2.79, 95% CI=1.93–4.04). The association between race and mortality did not vary significantly across causes of death (Pheterogeneity=0.202). However, the association between race and each of BC-specific and non-BC-specific mortality disappeared after adjusting for neighborhood socioeconomic deprivation, indicating that the racial disparity in BC-specific mortality and non-BC-specific mortality could be explained by neighborhood socioeconomic deprivation.
Discussion
Prior studies examining the prognostic influences of low socioeconomic condition or TNBC focus primarily on overall mortality and BC-specific mortality. To the best of our knowledge, this is the first multilevel competing-risk study to examine the associations of neighborhood socioeconomic deprivation and TNBC with BC-specific and non-BC-specific mortality in women with non-metastatic invasive BC. In a large, hospital-based cohort of BC patients, we found that neighborhood socioeconomic deprivation was significantly associated with greater risk of non-BC-specific mortality, independent of race and other sociodemographic factors, tumor pathology, treatment, comorbidity, smoking and alcohol use. The association between neighborhood socioeconomic deprivation and BC-specific mortality could be explained by sociodemographic factors rather than tumor characteristics, treatment, comorbidity or health behaviors (Supplementary Table). We also found that TNBC was independently associated with non-BC-specific mortality but not with BC-specific mortality, which approached significance. It is well-known that risks of both BC-specific and non-BC-specific mortality are higher among African American compared with white women. Our study provides evidence that the racial disparity in mortality could be explained by neighborhood socioeconomic deprivation.
Cancer registry-based studies have consistently reported associations between area-level socioeconomic deprivation and each of overall [2–7] and BC-specific mortality [8–10] among BC patients. But some studies suggest that the effect of area-level socioeconomic deprivation on mortality can be partly explained by ER-negative status [8], advanced stage [2, 4] and type of surgery [2], while others report that BC-specific mortality was not explained by demographic, tumor or treatment factors [3, 5–7, 10]. One potential reason for inconsistent results regarding the effect of area-level socioeconomic deprivation on mortality is that the analyses of population-based data from cancer registries may be insufficiently adjusted for potential individual-level confounders. Data from population-based cancer registries (e.g., SEER) are generally limited to information about tumor characteristics and only some cancer-related treatments (e.g., surgery and radiation therapy). In addition to demographic factors, tumor pathology, and local treatment, our study accounted for other individual-level factors that are not commonly found in population-based cancer registries, including data about HER2 amplification, health behaviors, type of health insurance, comorbidity, and systemic targeted therapies (chemotherapy, endocrine therapy, and trastuzumab). We found that the association of neighborhood socioeconomic deprivation with BC-specific mortality could be explained by sociodemographic factors rather than tumor characteristics or treatment.
Using a competing-risk analytic approach, we found a significant positive association between neighborhood socioeconomic deprivation and non-BC-specific mortality, which could not be fully explained by other variables in our models. In general, the cumulative probability of death from BC declines with age because of the increased risk of death from other causes [16, 30, 31]. Cardiovascular disease is the leading cause of death in elderly women with non-metastatic BC [16, 32]. Chemotherapy and trastuzumab have been associated with long-term cardiac toxicity in BC patients, particularly in older patients [33, 34]. Women with comorbidities also are more likely to die from other causes than BC [32, 35]. In addition, the probabilities of death from BC and from other causes were both found to be significantly higher in black compared with white patients with non-metastatic invasive BC diagnosed before 70 years of age [30]. However, our study showed that the association between neighborhood socioeconomic deprivation and non-BC-specific mortality could not be fully attributed to patient-level sociodemographics, tumor pathology, comorbidity, treatments, smoking status and alcohol use. Hence, research is needed to identify other environmental, behavioral, and genetic risk factors that might explain the association between area-level socioeconomic deprivation and non-BC-specific mortality.
Interestingly, our results showed that TNBC was significantly associated with increased risk of non-BC-specific mortality. Although the lack of targeted therapies for TNBC results in the worse BC-specific prognosis of TNBC than other BC subtypes [15], high-dose chemotherapy has been found to be more effective in TNBC than luminal A subtype [36]. We found that the association between TNBC and BC-specific mortality approached significance in the adjusted multilevel Cox proportional-hazards model. This observation is likely due to smaller numbers of TNBC cases in each group when we split the sample by cause of death for the competing-risk analysis.
Our study had several strengths. First, given an intra-correlation between participants nested within the same neighborhood, we used a multi-level proportional hazards model to characterize the respective influence of neighborhood socioeconomic deprivation and TNBC on BC mortality. Ignoring structural features of this type of data potentially biases the parameter estimates [22]. Second, prior studies examining socioeconomic status and breast cancer outcomes did not account for causes of death other than BC as competing risks, which could result in the overestimation of the effects of these factors on mortality [24]. We applied a multilevel proportional-hazards modeling approach integrated with the competing risk to compare differential effects of neighborhood socioeconomic deprivation on BC-specific and non-BC-specific mortality in a single statistical model. We found that neighborhood socioeconomic deprivation was independently associated with non-BC-specific mortality but not with BC-specific mortality. In addition, we computed a composite neighborhood socioeconomic deprivation index at the census-tract level, which captures multiple domains of disadvantage and yields a more robust indicator of neighborhood socioeconomic deprivation than a single indicator [22].
Our study also had some limitations. Data for the study sample were retrieved from a tumor registry at a single institution, which is the only National Cancer Institute-designated comprehensive cancer center in Missouri. Therefore, our results may reflect selection bias, which limits the generalizability of our findings. Further population-based studies are necessary to evaluate the extent of any selection bias and to validate our findings. However, our hospital-based registry provides rich data about comorbidity, HER2 amplification as well as ER- and PR-receptor status, targeted therapies, health insurance, smoking status and alcohol use, which are generally unavailable in large, population-based cancer registries. Single-center studies also can benefit from having centralized pathological testing and reporting systems and provision of similar treatment and follow-up (i.e., standards of care) for BC patients eliminating the confounding effect of between-hospital variations in standards of care and BC outcomes. Provision of similar treatment and follow-up could explain the lack of racial differences in survival outcomes observed in studies of TNBC patients treated at comprehensive cancer centers [37, 38]. Our study suggests that diagnosis, treatment, and follow-up provided at our institution did not substantially explain the association between neighborhood socioeconomic deprivation and BC-specific mortality. Second, some patients might have moved after diagnosis, which could have affected our findings. However, most of our patients were older, and generally, older people are less likely to move than younger persons [22]. Additionally, we pooled all other causes of death together to strengthen statistical power for a competing-risk analysis. Future research with a larger sample should investigate the impact of neighborhood socioeconomic deprivation and TNBC on specific “other” causes of death in BC patients.
In conclusion, our study provides evidence that neighborhood socioeconomic deprivation was associated with non-BC-specific mortality in non-metastatic invasive BC patients, independent of patients’ sociodemographic characteristics, comorbidity, tumor characteristics, treatment, smoking status and alcohol use. In contrast, the association between neighborhood socioeconomic deprivation and BC-specific mortality could be explained by sociodemographic factors. TNBC was significantly associated with non-BC-specific mortality and its association with BC-specific mortality approached significance. Our findings suggest that strategies aimed at reducing these disparities in mortality among non-metastatic invasive breast cancer survivors are needed. Health care providers should pay closer attention to BC patients’ existing comorbidities and follow-up of treatment late effects, particularly in elderly patients and patients living in more socioeconomically deprived communities. A better understanding of the reasons for the influence of neighborhood socioeconomic deprivation and TNBC on prognosis could help reduce disparities in BC outcomes and improve prognosis of TNBC patients, who in our sample were more likely to live in the more socioeconomically deprived neighborhoods.
Supplementary Material
Acknowledgments
This study was supported in part by research funding from the National Cancer Institute at the National Institutes of Health (R01 CA109675, R01 CA102777). Dr. Lian also was supported in part by a Career Development Award from the National Cancer Institute (K07 CA178331). We thank Ms. Irene Fischer and Mr. James Struthers for data management and the preparation of neighborhood socioeconomic variables; their services were provided through the Health Behavior, Communication and Outreach Core, which is funded in part by the National Cancer Institute Cancer Center Support grant (P30 CA091842) to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, Missouri.
Footnotes
All authors declared no conflict of interests.
References
- 1.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. President’s Cancer Panel. 2003–2004 Annual Report. [Accessed October 30 2013];Living beyond cancer: finding a new balance. 2004 May; (Available at: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp03-04rpt/Survivorship.pdf)
- 2.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 3.Downing A, Prakash K, Gilthorpe MS, Mikeljevic JS, Forman D. Socioeconomic background in relation to stage at diagnosis, treatment and survival in women with breast cancer. British journal of cancer. 2007;96:836–840. doi: 10.1038/sj.bjc.6603622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastiaannet E, de Craen AJ, Kuppen PJ, Aarts MJ, van der Geest LG, et al. Socioeconomic differences in survival among breast cancer patients in the Netherlands not explained by tumor size. Breast Cancer Res Treat. 2011;127:721–727. doi: 10.1007/s10549-010-1250-z. [DOI] [PubMed] [Google Scholar]
- 5.Akinyemiju TF, Soliman AS, Johnson NJ, Altekruse SF, Welch K, et al. Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. Journal of cancer epidemiology. 2013;2013:490472. doi: 10.1155/2013/490472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannenbaum SL, Koru-Sengul T, Miao F, Byrne MM. Disparities in survival after female breast cancer diagnosis: a population-based study. Cancer Causes Control. 2013;24:1705–1715. doi: 10.1007/s10552-013-0246-5. [DOI] [PubMed] [Google Scholar]
- 7.Markossian TW, Hines RB, Bayakly R. Geographic and Racial Disparities in Breast Cancer-Related Outcomes in Georgia. Health services research. 2014;49:481–501. doi: 10.1111/1475-6773.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson CS, Hole DJ, Twelves CJ, Brewster DH, Black RJ, et al. Prognostic factors in women with breast cancer: distribution by socioeconomic status and effect on differences in survival. Journal of epidemiology and community health. 2001;55:308–315. doi: 10.1136/jech.55.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, et al. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005) Cancer Epidemiol Biomarkers Prev. 2009;18:121–131. doi: 10.1158/1055-9965.EPI-08-0679. [DOI] [PubMed] [Google Scholar]
- 10.Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, et al. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117:1542–1551. doi: 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross SK, Harris J, Recht A. Race, Socioeconomic status, and breast carcinoma in the US: what have we learned from clinical studies. Cancer. 2002;95:1988–1999. doi: 10.1002/cncr.10830. [DOI] [PubMed] [Google Scholar]
- 12.Rutqvist LE, Bern A Stockholm Breast Cancer Study G. Socioeconomic gradients in clinical stage at presentation and survival among breast cancer patients in the Stockholm area 1977–1997. Int J Cancer. 2006;119:1433–1439. doi: 10.1002/ijc.21949. [DOI] [PubMed] [Google Scholar]
- 13.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 2009;18:883–893. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 14.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 15.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, et al. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009;20:1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 16.Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29:4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 17.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103:1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Archives of internal medicine. 2000;160:953–958. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 19.Ly BH, Nguyen NP, Vinh-Hung V, Rapiti E, Vlastos G. Loco-regional treatment in metastatic breast cancer patients: is there a survival benefit? Breast Cancer Res Treat. 2010;119:537–545. doi: 10.1007/s10549-009-0610-z. [DOI] [PubMed] [Google Scholar]
- 20.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 22.Lian M, Schootman M, Doubeni CA, Park Y, Major JM, et al. Geographic Variation in Colorectal Cancer Survival and the Role of Small-Area Socioeconomic Deprivation: A Multilevel Survival Analysis of the NIH-AARP Diet and Health Study Cohort. Am J Epidemiol. 2011;174:828–838. doi: 10.1093/aje/kwr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lian M, Struthers J, Schootman M. Comparing GIS-Based Measures in Access to Mammography and their Validity in Predicting Neighborhood Risk of Late-Stage Breast Cancer. PLoS One. 2012;7:e43000. doi: 10.1371/journal.pone.0043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 25.Allison PD. Survival analysis using SAS: a practical guide. 2. SAS Press; Cary, North Carolina: 2010. [Google Scholar]
- 26.Chaix B, Rosvall M, Lynch J, Merlo J. Disentangling contextual effects on cause-specific mortality in a longitudinal 23-year follow-up study: impact of population density or socioeconomic environment? Int J Epidemiol. 2006;35:633–643. doi: 10.1093/ije/dyl009. [DOI] [PubMed] [Google Scholar]
- 27.Piccirillo JF, Costas I, Claybour P, Borah AJ, Grove L, et al. The measurement of comorbidity by cancer registries. Journal of registry management. 2003;30:8–14. [Google Scholar]
- 28.Lunn M, McNeil D. Applying cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 29.Tamimi RM, Colditz GA, Hazra A, Baer HJ, Hankinson SE, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131:159–167. doi: 10.1007/s10549-011-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 31.Chapman JA, Meng D, Shepherd L, Parulekar W, Ingle JN, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100:252–260. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azim HA, Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011;22:1939–1947. doi: 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- 34.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 35.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Annals of internal medicine. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 36.Lonning PE. Poor-prognosis estrogen receptor- positive disease: present and future clinical solutions. Therapeutic advances in medical oncology. 2012;4:127–137. doi: 10.1177/1758834012439338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawood S, Broglio K, Kau SW, Green MC, Giordano SH, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27:220–226. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Racial differences in outcomes of triple-negative breast cancer. Breast Cancer Res Treat. 2013;138:281–289. doi: 10.1007/s10549-012-2397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.