Abstract
Ion channels play key roles in physiology. They function as protein transducers able to transform stimuli and chemical gradients into electrical signals. They also are critical for cell signaling and play a particularly important role in epithelial transport acting as gateways for the movement of electrolytes across epithelial cell membranes. Experimental limitations, though, have hampered the recording of ion channel activity in many types of tissue. This has slowed progress in understanding the cellular and physiological function of these channels with most function inferred from in vitro systems and cell culture models. In many cases, such inferences have clouded rather than clarified the picture. Here, we describe a contemporary method for isolating and patch-clamping renal tubules for ex vivo analysis of ion channel function in native tissue. Focus is placed on quantifying the activity of the epithelial Na+ channel (ENaC) in the aldosterone-sensitive distal nephron (ASDN). This isolated, split-open tubule preparation enables recording of renal ion channels in the close to native environment under the control of native cell signaling pathways and receptors. When combined with complementary measurements of organ and system function, and contemporary molecular genetics and pharmacology used to manipulate function and regulation, patch-clamping renal channels in the isolated, split-open tubule enables understanding to emerge about the physiological function of these key proteins from the molecule to the whole animal.
Keywords: Patch clamp, Isolated renal tubules, Ion channel recording, Collecting duct, ENaC
1 Introduction
Ion channels are key intrinsic membrane proteins. They are responsible for the diversity of electrical signaling found in every living cell. In addition, electrolytes and water, the latter of which follows the movement of electrolytes via osmosis, are transported across epithelial barriers of the integumentary system, gastrointestinal tract, the kidney, secretory glands, and many other organs, in part, via ion channels. Vectorial ion transport across epithelia is a consequence of the selective expression of ion channels and transporters in apical and basolateral membranes of epithelial cells. Understanding the basics of ion channel biophysics and function as well as regulation is critical to understanding the physiological role of these important proteins and how, when dysfunctional, they contribute to pathology. The recent elucidation of many human diseases caused by ion channel dysfunction provides fascinating insights into the diverse roles ion channels serve (1–9). However, our limited ability to record ion channel activity in native tissue has limited discovery of channel function in physiological and pathological conditions. This is particularly true for ion channels in epithelia, such as those expressed in renal tubule cells critical to the fine-tuning of the electrolyte and water concentrations of plasma and urine.
Most electrophysiological studies of ion channels have been performed in either cell culture or recombinant systems (10–13). Because in vivo function must be extrapolated from findings in these artificial systems, such studies often produce results that differ from the channels’ real function in native cells. Importantly, the patch-clamp method, which is often used to quantify channel activity in cultured cells, also provides an opportunity, when applied to native tissue, to explore the properties of ion channels in their more native environment. We describe here an isolated, split-open tubule preparation that is suitable for ex vivo analysis of renal ion channels. This preparation allows access to both the apical and basolateral membranes of tubule epithelial cells. Here we describe key aspects of this methodology, including mechanical isolation of the aldosterone-sensitive distal nephron, preparation of these tubules for patch-clamp analysis, and the recording of ion channel activity in the cell-attached configuration. Focus is placed on recording the activity of the epithelial Na+ channel, ENaC, in the apical membrane of principal cells in the aldosterone-sensitive distal nephron.
The power of in vivo and ex vivo analysis of ion channel function is that it preserves the native setting and control of these critical proteins within the cell and retains many of the emergent properties inherent to real tissue. From these types of studies, structural and functional details, as well as understanding of regulation, can be obtained under normal and pathological conditions. Moreover, combining this readout of channel activity with genetically altered mice enables definition and clarification of the in vivo function of proteins that have been studied in vitro (14–18). In addition to being a tool for understanding the physiological function of particular proteins, mutant mice can also be used to model human diseases. Applying ex vivo analysis of ion channel function to preparations prepared from these animals then also enables the exploration of ion channels as causative agents and/or targets for disease.
Here we focus on a murine kidney preparation. However, this approach can be, and has been, used to study renal channels in other mammals. For instance, we have successfully used this approach with minor changes to investigate ENaC in rat and canine kidneys (unpublished data). In combination with other contemporary cell biology methods, measurements of organ and system function, and incorporation of genetically modified animals, this electrophysiological approach represents a powerful tool to study electrolyte transport in specific nephron segments, and enables precise understanding of the physiological role of specific channel proteins along the nephron (19–23). Knowledge gained from such studies in recent years has been instrumental in increasing our understanding of basic and clinical aspects of renal disease.
2 Materials
2.1 Mouse Sacrifice and Kidney Isolation
Six-week-old C57BL/6J male mice (this mouse strain is broadly commercially available, for instance from Jackson Laboratory, USA; strain code 000664) (see Note 1).
CO2 gas tank and hermetic chamber for sacrifice.
Cold Hanks balanced salt solution (HBSS) (Sigma Aldrich, USA).
6-cm-diameter plastic Petri dishes (BD Falcon, USA).
Standard straight forceps (Fine Science Tools, USA).
Straight surgical scissors (Fine Science Tools, USA).
Nitrile gloves (Kimberly-Clark, USA).
2.2 Isolation of Renal Tubules
Cold HBSS (Sigma Aldrich, USA).
One freshly harvested kidney kept on ice in HBSS.
10-cm-diameter plastic Petri dishes (TPP, Fisher Scientific, USA).
Single-edge steel blade (American line, USA).
6-cm-diameter plastic Petri dishes (BD Falcon, USA).
Two Dumont #4 forceps (Fine Science Tools, USA).
Stereo microscope (Nikon SMZ 645, Melville, NY, USA) (see Note 2).
18 × 18—Cover glass #2 cover glass (Fisher Scientific, USA) cut into 5 × 5 mm chips.
Diamond scriber with diamond tips (Techni-Tool, USA).
0.01% solution of poly-D-lysine (Sigma Aldrich, USA).
2.3 Single-Channel Analysis of ENaC Activity in Isolated Tubules Using the Patch-Clamp Method
Patch clamp amplifier (we use Axopatch 200B Molecular Devices., Downingtown, PA, USA; see Note 3).
Digitizer, i.e., Digidata 1322A or 1400 A/D board (Molecular Devices) interfaced with a PC running appropriate data acquisition and analysis software (i.e., pClamp 9.2 or newer software suite from Molecular Devices).
Precision micromanipulator (we use MP-285 from Sutter Instr. Co., Novato, CA, USA) and mechanical micromanipulator (i.e., NMN-21 Narishige, East Meadow, NY, USA).
Vibration isolation table with Faraday cage (i.e., Tech. Manufacturing Co., Peabody, MA, USA).
Inverted microscope (i.e., Nikon TE2000-U, Melville, NY, USA).
Micropipette puller (i.e., Model P-97 Flaming/Brown puller, Sutter Instrument Co., USA).
Micro-forge (MF-830 Narishige, East Meadow, NY, USA).
Borosilicate glass capillaries (World Precision Instruments, Sarasota, FL, USA) pulled and forged to 7–10 mΩ for cell-attached patch-clamp recording.
Fast exchange recording/perfusion chamber (we use model RC-22, Warner Instruments, USA).
Multichannel valve perfusion system (i.e., Valve Bank II, AutoMake Scientific, USA).
Pipette solution: 140 mM LiCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.4) (see Note 4).
Extracellular bathing solution: 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 5 mM glucose, and 10 mM HEPES (pH 7.4) (see Note 4).
Adjustable volume pipette (10–100 μL) with appropriate tips (Eppendorf Research plus 100 μL, Eppendorf, USA) for drug application.
Eight-pole low-pass Bessel filter (LPF-8, Warner Instr. Corp. Hamden, CT, USA).
3 Methods
Isolating tubules for patch-clamp analysis has much in common with isolating them for perfusion and microelectrode work (19, 24–27). Similarly, patch-clamp analysis of channels in native cells of isolated tubules is similar to that of immortalized and freshly isolated cells held in culture (28–33).
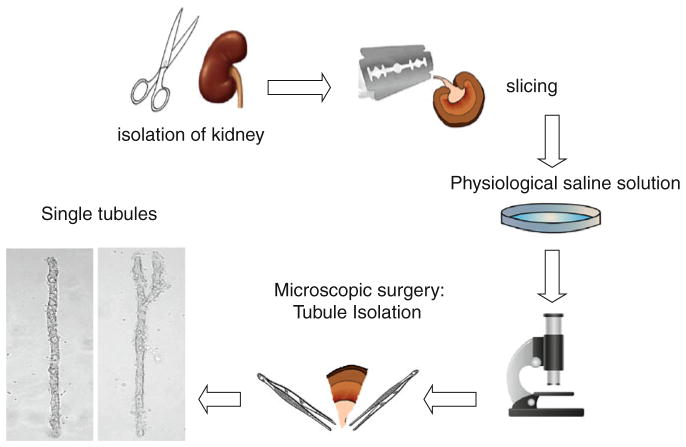
To be able to study ENaC in ASDN isolated from normal and genetically altered mice, the technique used to isolate tubules was modified to allow patch clamping of the apical plasma membrane of tubule cells. This modification combines splitting open the tubule followed by patch clamping of individual cells. In addition to the electrophysiological measurements described below, the modified approach for isolation may be useful for several other applications, e.g., immunohistochemical, biochemical, and molecular analysis of this tissue. Figure 1 illustrates the steps required to mechanically isolate tubules from the kidney without enzymatic treatment. Opening of the tubule with two micropipettes is shown in Fig. 2. Enzymatic techniques have been commonly used for the preparation of isolated tubules as a model for many biochemical and physiologic investigations. However, they are not recommended for electro-physiology study since changes during digestion may lead to drastic disturbances in protein function. Destroying the normal cellular environment and connections with other cells may also result in conditions different than normally seen in physiology. Thus, mechanical dissociation of tubules, which preserves tissue and cellular integrity protecting native structure and surroundings, is recommended.
Fig. 1.
Schematic illustration of the method used to isolate cortical collecting ducts for patch-clamp analysis. The kidney is isolated from the mouse and then cut into thin slices (<1 mm). Segments of interest are then mechanically isolated from these slices with microdissection typically using watchmaker forceps and a stereomicroscope
Fig. 2.
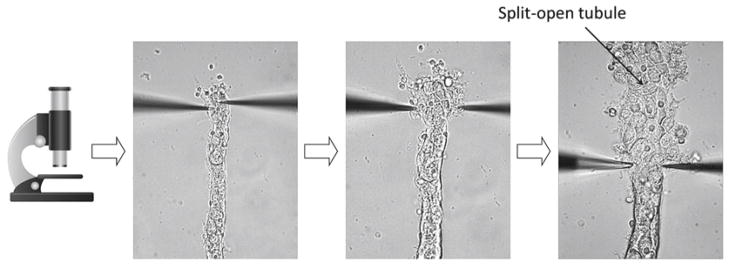
Shown here is a segment of the cortical collecting duct. The top of this segment has been split open to allow patch-clamp access to the apical membranes of lining epithelial cells
There are four main patch-clamp configurations: cell attach, inside-out, outside-out, and whole cell (34) (See also Chapter 7 of this book). We mostly use in our experiments the cell-attached configuration. However, these other configurations are also compatible with this preparation. With the cell-attached configuration, as shown in Fig. 3a, the intracellular face of the channel is exposed to the native intracellular milieu and the extracellular face to solution in the recording pipette. Figure 3b shows ENaC current traces from a cell-attached patch of a native principal cell in a freshly isolated mouse ASDN. The patched membrane was presented with test potentials that ranged from 0 to −60 mV. This seal contained at least three ENaC channels. Figure 3c shows the single-channel current–voltage relation for ENaC in mouse principal cells in freshly isolated ASDN. Similar current–voltage relationships are observed in rat tubules (32, 35–37). From this relationship, the conductance of ENaC can be determinate. In cell-attached patches, ENaC has a conductance of 4–5 pS with 140 mM NaCl in the pipette (38). To increase channel conductance without affecting any other channel property, the permeant ion in the patch pipette, Na+, was replaced by Li+. The latter permeates through ENaC better, increasing channel conductance.
Fig. 3.
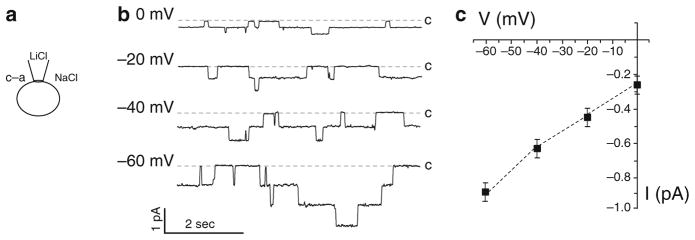
Recording ENaC at the apical membrane of a principal cell in an isolated, split-open collecting duct. (a) Patch-clamp recording in cell-attached con fi guration. Major ions in bath and pipette solutions are shown. (b) Representative single-channel current traces for ENaC from a principal cell. All recordings were performed in the cell-attached configuration in the voltage-clamp mode. Current was recorded at test potentials that ranged from 0 to −60 mV. Inward Li+ currents are depicted as downward deflection, and the dashed lines show the 0 current level (closed state) at each voltage. (c) Single-channel current–voltage relationship for ENaC in cell-attached patches that were made on the apical membrane of principal cells in isolated split-open mouse collecting ducts
As shown in Fig. 4 the effect of acute application of regulatory factors on ENaC activity can be studied in freshly isolated renal tubules. Figure 4 shows representative current traces for ENaC in native principal cells before and after application of 1 μM of arginine vasopressin (AVP) (Fig. 4a) and 10 μM ATP (Fig. 4b). As is clear in these representative current traces and as we previously demonstrated, AVP increases ENaC activity (39–44). In contrast, ATP decreases ENaC activity (31, 45, 46).
Fig. 4.
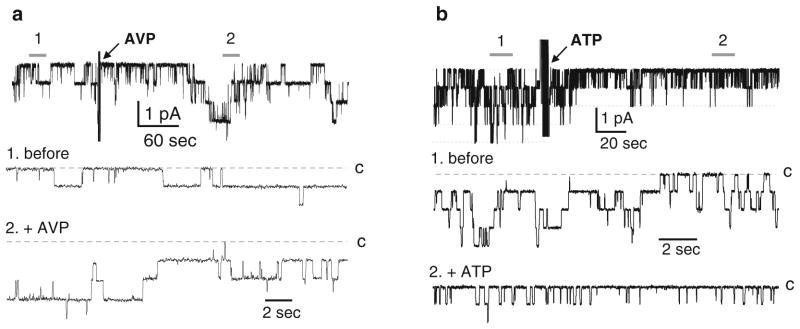
Activation of ENaC in principal cells of isolated cortical collecting ducts by arginine vasopressin (AVP) (a) and inhibition by ATP (b). Shown here are representative single-channel current traces for ENaC in a cell-attached patch from a principal cell before and after application of 1 μM AVP (a) and 10 μM ATP (b). Arrows indicate addition of AVP and ATP to the external bath solution. These patches were clamped to a holding potential of −Vp = −60 mV. Closed state noted with c and areas below 1 (before) and 2 (after addition of AVP or ATP) shown at an expanded time scale below. Data originally presented in (39, 46) and reproduced here with permission
The mechanism of ENaC regulation by dietary salt intake was studied in isolated tubules from animals maintained with different salt diets for at least 1 week (14–16). Experiments were performed on mice (Fig. 5) and rats (Fig. 6) kept on low (<0.01%) Na+, regular (0.32%) Na+, and high (2%) Na+ diets. As clear from the current traces in Figs. 5a and 6a, ENaC activity is inversely related to dietary salt intake. In addition to revealing biophysical characteristics, single-channel resolution also provides information about the number of active channels within a patched membrane (N) and the average probability that a channel will be open, commonly referred to as open probability (Po). Channel activity is routinely reported as NPo. In addition to N, information can be gleamed about channel density by quantifying the frequency (f) of observing a channel where f = patches with at least one active channel of that type/total number of viable seals for that condition. As demonstrated in Figs. 5b and 6b salt diet affected both Po and N.
Fig. 5.
ENaC activity and density are controlled by dietary NaCl intake in mice. (a) Representative current traces from cell-attached patches containing ENaC from mice kept on low (<0.01%)-Na+ (top), regular (0.32%)-Na+ (middle), and high (2%)-Na+ (bottom) diets. C denotes the closed state. (b) Summary of ENaC Po, fNPo, and N for mice kept on low, regular, and high-salt diets. *P < 0.05 vs. regular (0.32%)-Na+ diet; data are expressed as means ± SE. Numbers inside bars indicate number of experiments. Figure originally presented in (45) and reproduced here with permission
Fig. 6.
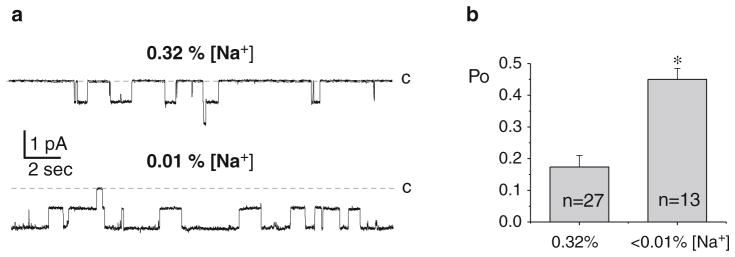
ENaC activity and density are controlled by dietary NaCl intake in rats. (a) Representative current traces from cell-attached patches containing ENaC from Sprague–Dawley rats kept on low (<0.01%)-Na+ (bottom) and regular (0.32%)-Na+ (top) diets. C denotes the closed state. (b) Summary of ENaC Po for rats kept on low (<0.01%)-Na+ and regular (0.32%)-Na+ diets. *P < 0.05 vs. regular (0.32%)-Na+ diet; data are expressed as means ± SE. Numbers inside bars indicate number of experiments. Figure originally presented in (32) and reproduced here with permission
Successful isolation and splitting of renal tubules and electro-physiological analyses is detailed as described below:
3.1 Mouse Kidney Isolation
Make sure that your handling of animals adheres to the appropriate ethical guidance and legislation (see Note 5).
Animal work is performed using protective gloves under appropriate safety conditions.
6–8-week-old male mice are sacrificed by CO2 administration followed by cervical dislocation (see Note 6).
The animal is placed on a surgical table. Using forceps and scissors make an incision exposing the chest cavity. Retractors can be used to keep the incision open (see Note 7).
The kidney is then removed and immediately put on ice in a 6-cm-diameter plastic Petri dish with approximately 4 mL of the HBSS solution.
3.2 Isolation of Mouse Renal Tubules
The kidney is taken from the HBSS solution and decapsulated using forceps. Figure 1 illustrates the steps of this procedure for reference.
The decapsulated kidney is cut into thin slices (<1 mm) with a razor blade in a 10-cm-diameter plastic Petri dish.
One of the slices is placed into a new 6-cm-diameter plastic Petri dish with approximately 3 mL of fresh ice-cold HBSS solution. This Petri dish is put under the stereomicroscope.
Using two watchmaker forceps the slice is divided into sections using blunt dissection. Each section should contain approximately 10–20 tubules. From the cortical part of each section, the collecting tubules of individual nephrons are carefully isolated (see Note 8).
Isolated tubules are transferred to poly-D-lysine pre-coated 5 × 5 mm glass chips (see Note 9).
3.3 Splitting Open Tubules
A chip containing tubules is placed into the recording chamber affixed to the stage of an inverted microscope. The chip is perfused with bath (extracellular) solution for 2–3 min to remove cell debris (see Note 10).
The tubule is then split open with two sharp micropipettes controlled with separate micromanipulators to gain access to the apical plasma membrane of epithelial cells within the tubule.
The tubule is oriented in the chamber so that its midline is perpendicular to the front of the microscope.
The tubule is gently pinned with the left micropipette, taking care not to press through to the bottom cells. With the left pipette stationary, the top surface of the tubule is scraped away with the right pipette to open it (see Fig. 2).
When the tubule is opened the left pipette is moved a small distance closer to the front, and step number 4 is repeated. Continue to split open the tubule as needed for your experiment.
The apical membranes of cells within the tubule are cleaned with a suction pipette under microscopic observation. With this cleaning procedure, the success rate in obtaining gigaohm seals increases.
3.4 Single-Channel Analysis of ENaC Activity Using the Patch-Clamp Method
Prepare bath (extracellular) and pipette solutions, and patch pipettes (see Note 11).
The fire-polished glass patch pipette is slowly lowered to the surface of the selected cell (see Note 12) and a high-resistance (>1 gΩ) seal is formed between the pipette and plasma membrane by applying negative pressure to the back of the recording pipette usually through gentle suction. Seal formation is assessed by monitoring pipette resistance with resistance going from ~7–10 mΩ to more than 8–10 gΩ upon successfully forming the cell-attached seal.
The patch membrane can then be voltage clamped (keeping the voltage constant) to observe changes in current or current clamped (keeping current constant) to observe changes in membrane voltage. For our cell-attached voltage-clamp studies, currents were low-pass filtered at 100 Hz by an eight-pole Bessel filter and digitized and stored on a PC hard drive using the Digidata interface (see Note 3).
Acknowledgments
Research from the author’s laboratories is supported by the NIH grants R01 DK59594, R01 DK087460, R01 DK070571 (to J.D.S.), and R01HL108880 (to A.S.).
Footnotes
It is also possible to use other mouse strains and gender. This approach also can be used to study renal channels in rat, rabbit, canine, and other mammals.
The stereomicroscope Nikon SMZ 645 is not produced anymore. However Nikon AMZ 745 or any other comparable model should be sufficient for tubule isolation.
There are many different patch-clamp amplifiers, A/D acquisition boards and programs, micromanipulators, isolation tables, microscopes, pipette pullers, perfusion chambers, etc. available for patch-clamp analysis. The user should use those best suited to their experiments and personal preference. For a detailed description of the patch-clamp method, the reader is directed towards the excellent book by B. Sakmann and E. Neher (34); see also Chapter 7 of this book.
The pipette and extracellular bathing solutions should be appropriate for the channel of interest. The choice of solutions depends on experiments conditions.
Non-survival surgeries are performed in septic but clean conditions. Instruments used should be clean, but not necessary sterile. All solutions used in this preparation should be sterile and kept on ice.
Carbon dioxide (CO2) inhalation is a common method of euthanasia used for rats and mice. Without pre-charging the chamber, place the animals in the chamber and introduce 100% CO2 at a slow rate so as to minimize distress. Animals should be exposed to CO2 for at least 5 min. It is also possible to use other euthanasia techniques. Upon completion of the procedure, death must be insured by cervical dislocation or other methods approved by the Institutional Ethics (or equivalent) Committee.
A retractor helps in keeping the incision open and to keep the skin and tissue away from organs. The user should use those best suited to their experiments and personal preference.
Isolated tubules can be identified by morphology under the stereomicroscope. The ASDN can be identified as merging of connecting tubule into cortical collecting duct. Proximal tubules in the cortex can be distinguished by their cloudy pale color and very broad structure. In contrast, distal elements (cortical ascending limb, distal convoluted tubule, cortical collecting duct, and loop of Henle) are more transparent and narrower. Cortical collecting duct can be readily distinguished by the ability to see individual cells within the tubule (26, 27).
Construct glass chips as follows: coverslips are placed onto a hard glass surface and held in place with plastic overlays. One coverslip is scored with a scriber pen with a diamond tip in a 4 × 4 grid. Scorings are tapped to separate chips. The resulting glass pieces are approximately 5 × 5 mm and are held in a 35 mm tissue culture dish until use. Cover glass chips are coated with poly-D-lysine a day before experiments. Poly-D-lysine is used to facilitate adherence of tubules to cover glass. This, in some instances, can be particularly important for patch-clamp experiments performed with constant bath perfusion. We do not recommend using coverslips with lysine coating that are more than 5–7 days old. Cell-Tak (BD Bioscience) or other adhesive materials can be used as alternative to poly-D-lysine.
Other chips with tubules should be kept on ice in a Petri dish in HBSS solution until needed. The number of chips and tubules depends on experimental needs, but the researcher should take into account that the experimental window for using freshly isolated tubules is less than 2–3 h.
The various configurations of the patch clamp technique may be used to record ion channel activity in renal tubules. The interior of the pipette should be filled with an appropriate solution. We use a standard physiological saline solution for the bath while performing cell-attached recordings. We recommend checking the osmolarity of pipette solutions which should be 295 ± 5 mOsm. After preparation, the pipette solution should be aliquoted and stored in a freezer before experiments to prevent degradation of labile ingredients.
The collecting ducts contain two main cell types, principal and intercalated cells. For our study we used principal cells, which are the more abundant type.
References
- 1.Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 2.Bonny O, Hummler E. Dysfunction of epithelial sodium transport: from human to mouse. Kidney Int. 2000;57:1313–1318. doi: 10.1046/j.1523-1755.2000.00968.x. [DOI] [PubMed] [Google Scholar]
- 3.Hummler E, Horisberger JD. Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human diseases. Am J Physiol. 1999;276:G567–G571. doi: 10.1152/ajpgi.1999.276.3.G567. [DOI] [PubMed] [Google Scholar]
- 4.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- 5.Schild L. The ENaC channel as the primary determinant of two human diseases: Liddle syndrome and pseudohypoaldosteronism. Nephrologie. 1996;17:395–400. [PubMed] [Google Scholar]
- 6.Schild L. The epithelial sodium channel: from molecule to disease. Rev Physiol Biochem Pharmacol. 2004;151:93–107. doi: 10.1007/s10254-004-0023-7. [DOI] [PubMed] [Google Scholar]
- 7.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP. Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 8.Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle’s syndrome mutations increase activity of a human epithelial Na+ channel. Cell. 1995;83:969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 9.Wollnik B, Schroeder BC, Kubisch C, Esperer HD, Wieacker P, Jentsch TJ. Pathophysiological mechanisms of dominant and recessive KVLQT1 K+ channel mutations found in inherited cardiac arrhythmias. Hum Mol Genet. 1997;6:1943–1949. doi: 10.1093/hmg/6.11.1943. [DOI] [PubMed] [Google Scholar]
- 10.Berjukow S, Doring F, Froschmayr M, Grabner M, Glossmann H, Hering S. Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br J Pharmacol. 1996;118:748–754. doi: 10.1111/j.1476-5381.1996.tb15463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamper N, Stockand JD, Shapiro MS. The use of Chinese hamster ovary (CHO) cells in the study of ion channels. J Pharmacol Toxicol Methods. 2005;51:177–185. doi: 10.1016/j.vascn.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Robbins J, Trouslard J, Marsh SJ, Brown DA. Kinetic and pharmacological properties of the M-current in rodent neuroblastoma × glioma hybrid cells. J Physiol. 1992;451:159–185. doi: 10.1113/jphysiol.1992.sp019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staruschenko A, Booth RE, Pochynyuk O, Stockand JD, Tong Q. Functional reconstitution of the human epithelial Na+ channel in a mammalian expression system. Methods Mol Biol. 2006;337:3–13. doi: 10.1385/1-59745-095-2:3. [DOI] [PubMed] [Google Scholar]
- 14.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol. 2012;302:C188–C194. doi: 10.1152/ajpcell.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem. 2011;286:1054–1060. doi: 10.1074/jbc.M110.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283:36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE. Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol. 2012;302:F78–F84. doi: 10.1152/ajprenal.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubera I, Hummler E, Beermann F. Transgenic mice and their impact on kidney research. Pflugers Arch. 2009;458:211–222. doi: 10.1007/s00424-008-0624-0. [DOI] [PubMed] [Google Scholar]
- 19.Bailey MA, Giebisch G, Abbiati T, Aronson PS, Gawenis LR, Shull GE, Wang T. NHE2-mediated bicarbonate reabsorption in the distal tubule of NHE3 null mice. J Physiol. 2004;561:765–775. doi: 10.1113/jphysiol.2004.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang DY, Osswald H, Vallon V. Sodium reabsorption in thick ascending limb of Henle’s loop: effect of potassium channel blockade in vivo. Br J Pharmacol. 2000;130:1255–1262. doi: 10.1038/sj.bjp.0703429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallon V. Micropuncturing the nephron. Pflugers Arch. 2009;458:189–201. doi: 10.1007/s00424-008-0581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol. 2001;281:F1117–F1122. doi: 10.1152/ajprenal.2001.281.6.F1117. [DOI] [PubMed] [Google Scholar]
- 24.Kirchner KA. Greater loop chloride uptake contributes to blunted pressure natriuresis in Dahl salt sensitive rats. J Am Soc Nephrol. 1990;1:180–186. doi: 10.1681/ASN.V12180. [DOI] [PubMed] [Google Scholar]
- 25.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension. 1991;17:1018–1024. doi: 10.1161/01.hyp.17.6.1018. [DOI] [PubMed] [Google Scholar]
- 26.Wagner CA, Lukewille U, Valles P, Breton S, Brown D, Giebisch GH, Geibel JP. A rapid enzymatic method for the isolation of defined kidney tubule fragments from mouse. Pflugers Arch. 2003;446:623–632. doi: 10.1007/s00424-003-1082-3. [DOI] [PubMed] [Google Scholar]
- 27.Schafer JA, Watkins ML, Li L, Herter P, Haxelmans S, Schlatter E. A simplified method for isolation of large numbers of defined nephron segments. Am J Physiol Renal Physiol. 1997;273:F650–F657. doi: 10.1152/ajprenal.1997.273.4.F650. [DOI] [PubMed] [Google Scholar]
- 28.Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through βPix/14-3-3/Nedd4-2. J Am Soc Nephrol. 2010;21:833–843. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlov TS, Ilatovskaya DV, Levchenko V, Mattson DL, Roman RJ, Staruschenko A. Effects of cytochrome P450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC) Am J Physiol Renal Physiol. 2011;301:F672–F681. doi: 10.1152/ajprenal.00597.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol. 2007;130:399–413. doi: 10.1085/jgp.200709800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol. 2008;294:F38–F46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- 32.Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na + channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol. 2007;18:1652–1661. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- 33.Yu L, Helms MN, Yue Q, Eaton DC. Single-channel analysis of functional epithelial sodium channel (ENaC) stability at the apical membrane of A6 distal kidney cells. Am J Physiol Renal Physiol. 2008;295:F1519–F1527. doi: 10.1152/ajprenal.00605.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakmann B, Neher E. Single-channel recording. 2. Springer; 1995. pp. 1–722. [Google Scholar]
- 35.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–F1070. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karpushev AV, Levchenko V, Ilatovskaya DV, Pavlov TS, Staruschenko A. Novel role of Rac1/WAVE signaling mechanism in regulation of the epithelial Na+ channel. Hypertension. 2011;57:996–1002. doi: 10.1161/HYPERTENSIONAHA.110.157784. [DOI] [PubMed] [Google Scholar]
- 37.Sun P, Lin DH, Yue P, Jiang H, Gotlinger KH, Schwartzman ML, Falck JR, Goli M, Wang WH. High potassium intake enhances the inhibitory effect of 11,12-EET on ENaC. J Am Soc Nephrol. 2010;21:1667–1677. doi: 10.1681/ASN.2009111110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer LG, Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci USA. 1986;83:2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol. 2009;297:F1411–F1418. doi: 10.1152/ajprenal.00371.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Williams SK, Schafer JA. Differences in synergistic actions of vasopressin and deoxycorticosterone in rat and rabbit CCD. Am J Physiol Renal Physiol. 1990;259:F147–F156. doi: 10.1152/ajprenal.1990.259.1.F147. [DOI] [PubMed] [Google Scholar]
- 41.Frindt G, Burg MB. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972;1:224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- 42.Frindt G, Palmer LG. Regulation of Na channels in the rat cortical collecting tubule: effects of cAMP and methyl donors. Am J Physiol Renal Physiol. 1996;271:F1086–F1092. doi: 10.1152/ajprenal.1996.271.5.F1086. [DOI] [PubMed] [Google Scholar]
- 43.Hawk CT, Li L, Schafer JA. AVP and aldosterone at physiological concentrations have synergistic effects on Na + transport in rat CCD. Kidney Int. 1996;57:S35–S41. [PubMed] [Google Scholar]
- 44.Helman SI, Grantham JJ, Burg MB. Effect of vasopressin on electrical resistance of renal cortical collecting tubules. Am J Physiol. 1971;220:1825–1832. doi: 10.1152/ajplegacy.1971.220.6.1825. [DOI] [PubMed] [Google Scholar]
- 45.Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J. 2010;24:2056–2065. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol. 2010;21:1903–1911. doi: 10.1681/ASN.2010040377. [DOI] [PMC free article] [PubMed] [Google Scholar]