Table 2.
Scope of the Asymmetric Synthesis of the Dihydropyran Derivates 4 via a One-Pot Domino Michael–Hemiacetalization and Dehydration Reaction
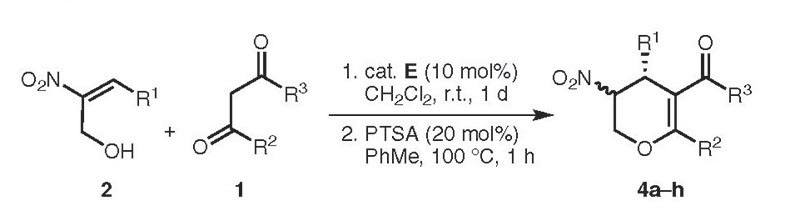
|
||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 4a | R1 | R2 | R3 | Yield (%)b | de (%)c (trans) | ee (%)d,e |
| 4a | Ph | Me | OMe | 80 | 71 | 95 (71) |
| 4b | Ph | Me | OEt | 85 | 64 | 88 (73) |
| 4c | Ph | Ph | OEt | 81 | 98 | 99 (99) |
| 4d | Ph | Me | Me | 77 | 87 | 92 (99) |
| 4e | 4-MeC6H4 | Me | OMe | 81 | 45 | 88 (89) |
| 4f | 3-BrC6H4 | Me | OMe | 86 | 76 | 88 (78) |
| 4g | 2-thienyl | Me | OMe | 82 | 26 | 90 (94) |
| 4h | 3,4-(OCH2O)C6H3 | Me | OMe | 81 | 43 | 91 (83) |
All reactions were performed on a 1.0 mmol scale.
Yield of isolated product.
Determined by HPLC analysis.
Determined by HPLC analysis on a chiral stationary phase.
The enantiomeric excess of the minor cis-diastereomer is given in parentheses.
