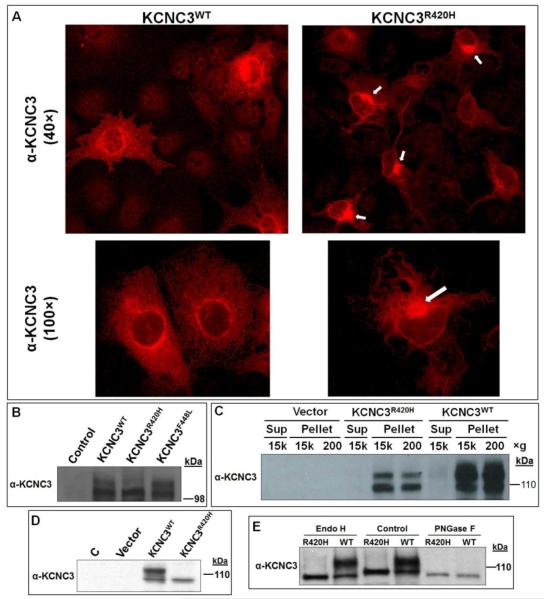
Figure 1. Localization and differential protein modification of KCNC3WT, KCNC3F448L, and KCNC3R420H in COS-1 and SH-SY5Y cells.
(A) OS-1 cells transiently transfected with either KCNC3WT or KCNC3R420H were analyzed by confocal fluorescence microscopy using a KCNC3 antibody. KCNC3WT shows perinuclear distribution and punctate staining of plasma membrane whereas KCNC3R420H displays primarily perinuclear staining (white arrows) and very limited fluorescence associated with the plasma membrane. Top and bottom panels were obtained at 40× and 100× magnification, respectively. (B) Immunoblot analysis of KCNC3 in COS-1 cells transiently transfected with human KCNC3WT, KCNC3F448L, or KCNC3R420H expression vectors illustrates a significant reduction of a slower migrating (upper) band consistent with altered posttranslational modifications. (C) Immunoblot analysis of total protein from COS-1 cells transiently transfected with KCNC3WT or KCNC3R420H subjected to differential centrifugation verifies that there was no fractional loss of the KCNC3R420H protein within our isolation protocol and illustrates the diminished upper band. (D) Immunoblot analysis of KCNC3 in stably transfected human neuroblastoma SH-SY5Y cells illustrating the complete absence of the upper band. (E) Immunoblot analysis of KCNC3 in stably transfected SH-SY5Y cells was used to evaluate the treatment of total protein with Endo H or PNGase F. The upper band was resistant to Endo H whereas this species disappeared when total cell extracts were subjected to PNGase F treatment, consistent with the removal of a complex glycan. Both enzymes however led to cleavage of the lower band (control lanes) generating a faster migrating band presumably due to release of a secondary high mannose modification as further illustrated in Figure S1. The removal of a high mannose moiety is consistent with the known activity of Endo H.